Custom Camelid Fc-Fusion Protein Preparation Service
Camelid Fc-Fusion Protein Preparation Feature Case Study Published Data FAQ Resources
Creative Biolabs has been a long-standing expert in VHH development. With over ten years of extensive experience and advanced antigen preparation platforms, our scientists are confident in designing and preparing optimized camelid
Fc-fusion proteins as immunogens tailored to meet your specific project needs. By partnering with Creative Biolabs, our specific immunogen preparation strategies will enhance your VHH development programs and increase the potential for research success.
Camelid Fc-Fusion Protein Preparation at Creative Biolabs
Creative Biolabs is a well-recognized expert in high-quality antigen preparation for global customers. Selecting the proper immunogen is one of the most critical steps in a successful single-domain antibody (sdAb) development project. For target proteins
that are small or have low immunogenicity, these immunogens may need to be designed and prepared in the optimized formats (such as Fc-fusion) for immunization. However, human Fc or mouse Fc, often used to increase antigen size or exposure, may lead
to tag immunogenicity and reduce the possibility of in vivo VHH production against targets.
With advanced platforms and powerful technologies, our scientists are confident in overcoming these challenges to meet the demands of our global customers. We have more than ten years of extensive experience in providing a unique series of camelid (e.g., llama, alpaca, and camel) Fc-fusion proteins for immunogen preparation, ensuring a promising immune response in the camelid immunization stage. Whether you have predetermined protocols or need Creative Biolabs to develop one, our professional staff
will work closely with you to ensure the success of your program. We offer technical support with exceptional reliability and the fastest turnaround time.

Features of Camelid Fc-Fusion Proteins
-
Enhanced target protein immunogenicity
-
Long half-life and good stability
-
Much lower tag immunogenicity and low endotoxin level
-
High purity and activity
In recent years, there has been a dramatic increase in global interest and sales of therapeutic VHHs. As a pioneer and global leader in VHH development, Creative Biolabs is professional in applying advanced strategies for camelid
Fc-fusion protein preparation to satisfy various project demands. If you are interested in our services, please do not hesitate to contact us for more details. We look forward to discussing your
inquiry and finding the best solution for your needs.
Published Data
-
Specific VHHs and VHH-Fc Fusion Antibodies Neutralize Toxic α–Cbtx and Improve Survival Rates in Mice
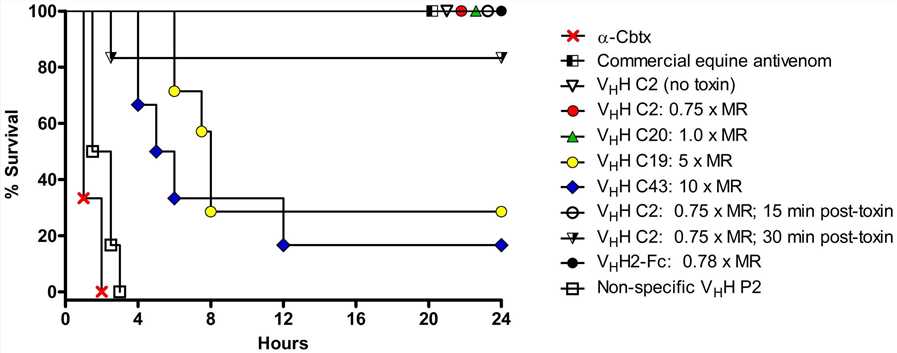 Fig. 1 Various α–Cbtx-specific VHHs and VHH2-Fc antibodies reduced lethality induced by α–Cbtx in mice.1
Fig. 1 Various α–Cbtx-specific VHHs and VHH2-Fc antibodies reduced lethality induced by α–Cbtx in mice.1
This study examines the ability of llama-derived single-domain antibodies (VHHs) and a VHH-Fc fusion antibody to neutralize α-cobratoxin (α-Cbtx) in vivo. Researchers immunized llamas, identified high-affinity VHHs, created a VHH-Fc fusion
antibody, and produced it in plants. As illustrated in Fig.1, the efficacy of various α–Cbtx-specific VHHs and a VHH2-Fc antibody in neutralizing α-Cbtx-induced lethality was assessed in mice. The results showed that all of the specific VHHs and VHH2-Fc
provided either prolonged survival or complete protection against the α-Cbtx challenge when co-administered. The most efficacious antibodies were VHH C2 and VHH2-Fc, which neutralized α–Cbtx at 0.75× and 0.78× MR (molar ratio) of VHH to toxin, respectively,
demonstrating 100% survival and total neutralization of α-Cbtx. These newly discovered antibodies were found to be effective in counteracting the lethal effects of α-Cbtx in vivo, potentially paving the way for the development of new antidotes
for snake venom.
Reference
-
Richard, Gabrielle, et al. "In vivo neutralization of α-cobratoxin with high-affinity llama single-domain antibodies (VHHs) and a VHH-Fc antibody." PloS one 8.7 (2013): e69495. Distributed under Open Access license CC BY 4.0, without modification.
FAQ
1. What is Camelid Fc-Fusion Protein?
Camelid Fc-fusion proteins consist of the Fc (fragment crystallizable) region of camelid antibodies combined with other protein domains, such as the variable regions of antibodies or functional proteins.
2. What are the advantages of using Fc-fusion proteins in immunogen preparation?
Fc-fusion proteins combine the high specificity of the targeting domain with the benefits of the Fc region. The Fc region enhances solubility, stability, and half-life. This leads
to improved immunogenic potential, stronger immune responses, and simplified protein purification.
3. How is a Camelid Fc-Fusion Protein constructed for use as an immunogen?
Constructing a Camelid Fc-fusion protein involves several steps. Firstly, the gene encoding the target of interest is fused in-frame with a gene encoding the Camelid Fc region. This construct is then inserted into an appropriate expression vector,
which is introduced into a suitable host cell line (like CHO or HEK293 cells) for protein expression.
4. How to verify the functionality of a prepared Camelid Fc-Fusion Protein?
Binding assays such as enzyme-linked immunosorbent assay (ELISA), surface plasmon resonance (SPR), and bio-layer interferometry (BLI) can confirm the interaction between the Camelid Fc-fusion target and its confirmed ligand(s). SDS-PAGE and Western Blot can assess the size and integrity of the fusion target. Furthermore, functional cell-based assys can be performed to verify biological activity.
Resources
We are offering highly customized CRO services to assist your Single Domain Antibody (sdAb) related projects. Please Contact Us for more details.


 Fig. 1 Various α–Cbtx-specific VHHs and VHH2-Fc antibodies reduced lethality induced by α–Cbtx in mice.1
Fig. 1 Various α–Cbtx-specific VHHs and VHH2-Fc antibodies reduced lethality induced by α–Cbtx in mice.1
















