New drugs must undergo lengthy and rigorous studies and tests before being permitted for use in humans. In this connection, the human in vitro system plays a critical role in drug discovery, which involves the identification of a therapeutic target for a specific disease as a starting point and compound screening on the target acting and the validation followed by. Most of the entire work is manipulated using the outside human body system.
In vitro human systems refer to an experimental model that simulates various physiological environments and biochemical processes in the human body. Usually, carrying out experiments requires simulating human cells or tissues to observe various biochemical reactions in the human body in a specific culture environment. These test systems include cultures of single cell types, co-cultures of multiple cell types, three-dimensional culture systems, etc.
Drugs can be screened, validated, and optimized via in vitro human systems that, through nucleic acid detection, protein detection, live cell imaging, cell function testing, and other methods, can analyze and evaluate drug candidates' impact on the target and can also simulate the distribution, metabolism, and toxic effects of drugs in the body.
In vitro human systems have some unique advantages over cellular and animal models:
* They can better simulate the human microenvironment and tissue structure.
* For drug screening, in vitro human systems can study the effects on specific human cells or tissues.
* Researchers can precisely control experimental conditions, such as pH, temperature, and nutrients.
All in all, in vitro human systems can increase the efficiency of drug discovery, saving time and money.
In vitro human systems can be roughly divided into two-dimensional (2D) and three-dimensional (3D) models.
The 2D model is mainly based on lab-grown cell lines that are mainly made up of cells sensitive or resistant to a particular drug and can, to some extent, mimic the human body's response to a drug.
3D models, such as organ-on-a-chip and tissue slices, provide a research platform that is closer to the physiological environment and can be used to detect the effects of drugs on specific tissues and organs. The model can simulate the inter-tissue interaction, which is more accurate for assessing drug sensitivity, toxicity, and metabolism. For example, liver-on-a-chip models can simulate the real human liver environment for drug metabolism and toxicity studies.
These two systems are critical for early stage studies of drug discovery as they can save resources and improve the efficiency.
Monolayer cell culture methods are generally used to develop 2D models—human cells or cell lines are grown on 2D petri dishes or slides. This culture method is simple and efficient and is widely used in drug screening, toxicity testing, and gene function research.
However, there are some problems with 2D models:
* Cells tend to grow and differentiate in a 3D structure in the real biological environment.
* The 2D culture environment may not accurately simulate the real cell growth environment. For example, cells in 2D cell models may not establish normal intercellular communication, rendering predictions of drug effects in these models to deviate from reality.
Despite these problems, 2D cell models remain one of the most commonly used ones in modern biomedical research, mainly due to their simple operation, low cost, and suitability for large-scale screening.
For decades, researchers have been using 2D cell and animal models to study drug efficacy and toxicity. But both approaches have their limitations. Traditional cell cultures cannot simulate the complex tissue microenvironment. Yet animal models seem not to be an idealized way to study diseases since the disease and immune systems of animal models are different from those of humans. Efficacy and toxicity assessed in animal studies do not always predict actual clinical effects, and many drugs may fail at the clinical stage. In recent years, tissue engineering and 3D printing technology have made it possible to grow 3D tissue for drug screening.
3D models have many advantages:
There are different ways to construct 3D models, like stem cell differentiation and 3D printing.
Stem cell-derived 3D organoid systems are an avant-garde approach to pharmaceutical research. These systems primarily stimulate stem cells to differentiate into specific types of tissue cells through in vitro culture, which then organize into 3D tissue models that closely resemble real organ structures—referred to as "organoids". Stem cell-derived 3D organoid systems are created through the exploitation of stem cells' self-renewal and differentiation potential. Stem cells are cultivated in specialized culture media where the introduction of specific factors and chemicals incites their differentiation into specified tissue cell types.
What are the main operational steps of the stem cell-derived 3D organoid system technology?
1. Extract pertinent stem cells from the body, which are then placed in specific culture environments.
2. Stem cells are induced to differentiate and proliferate by tweaking the culture medium formula.
3. Upon reaching a certain cell count, they are either built into organ-like structures within a 3D scaffold or through special cell aggregation techniques.
4. They are then maintained in suitable culture conditions for further differentiation and maturation within the 3D structure, forming functional organ models.
3D organoid technology has been extensively applied in the construction of various organ models, including lungs, intestines, liver, kidneys, heart, and brain, providing novel tools for new drug development, and toxicity screening, as well as research in tissue engineering and regenerative medicine.
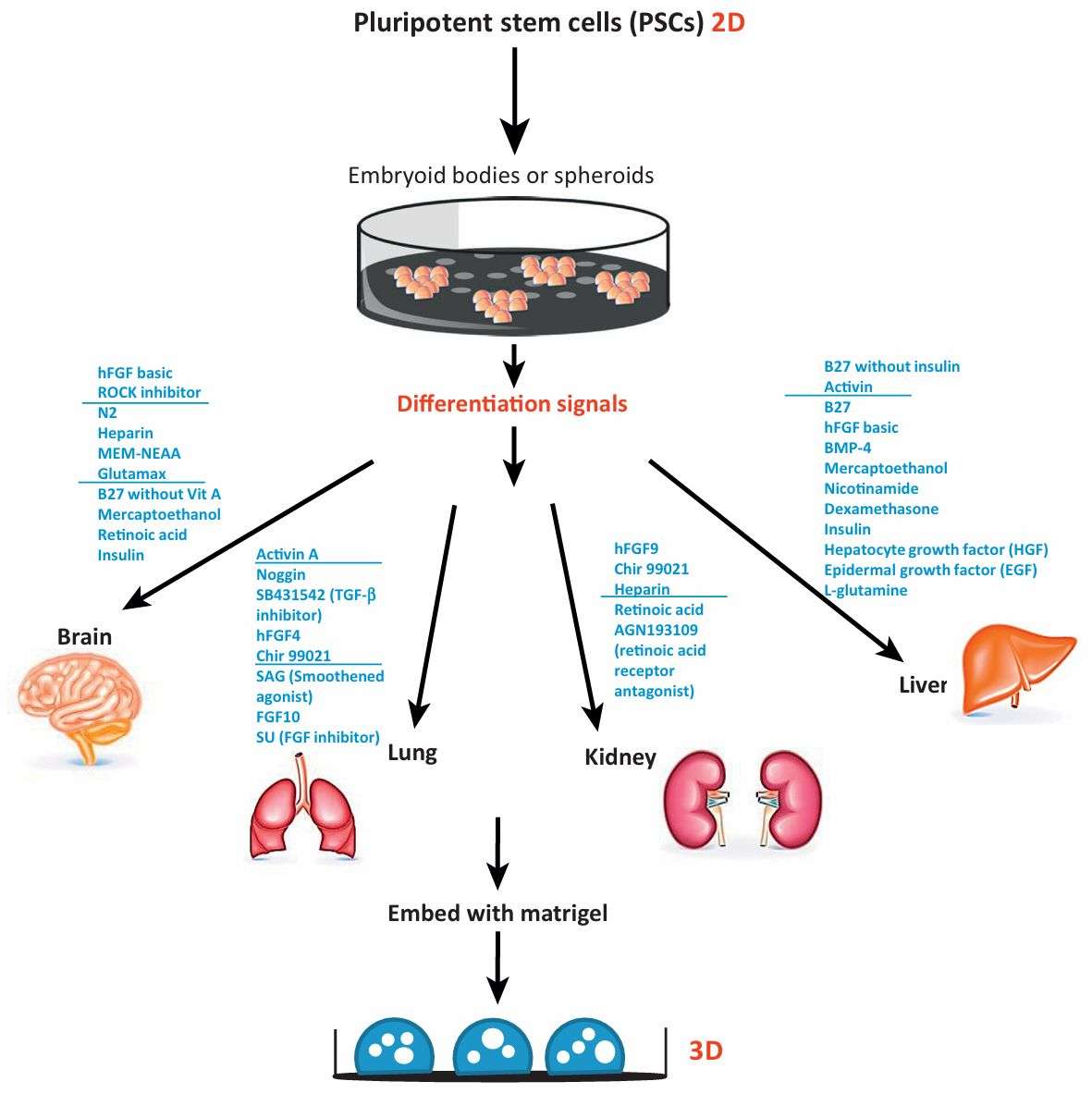 Fig. 1 Schematic diagram depicting current methods for generating organoids from PSCs. (Dutta Devanjali, 2017)
Fig. 1 Schematic diagram depicting current methods for generating organoids from PSCs. (Dutta Devanjali, 2017)
3D models bridge the gap between cell culture and in vivo models, as well as between animal models and human experiments. Bioprinting refers to the use of a computer-assisted transfer process to pattern and assemble living and non-living materials according to a set organization pattern to produce bioengineered structures for medical, biological, and pharmaceutical research. Cells must be maintained during the printing process, and this puts high demands on 3D printing technology—the sterility of the materials and equipment during the 3D printing process is critically demanded. Sterilization methods include the use of an autoclave, ultraviolet light, or ethanol, and bioprinting within a laminar flow hood.
In conclusion, bioprinting technology can precisely construct the desired model from the cellular to the organ level, improving efficiency and quality in drug discovery and disease model research.
Creative Biolabs offers a broad array of in vitro human system construction and testing services, including 2D cell culture and specialized 3D tissue models. Our expertise in constructing precise human 3D tissues and designing high-quality immunologic analysis assays has earned a strong reputation amongst our global customer bases.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.