Creative Biolabs offers camel anti-idiotypic antibody (single domain antibody) production service. Our R&D team has enriched experience in the selection of single domain antibodies with high affinity and specificity, production, and engineering. Camel is immunized with target antibodies in the form of whole IgG, scFv, Fab, or F(ab')2 that preserve the antibody activity to develop anti-idiotypic antibodies. Camel's RNA is extracted and reverse-transcribed to cDNA. With specific primers, genes encoding antibody heavy chains are amplified to create a cDNA antibody library. It is then efficiently packaged and displayed in M13 phages. High affinity and high specificity anti-ID antibodies could be isolated in 10-10 M range. Further affinity maturation and single domain antibody modification services are available at Creative Biolabs. Our staff are professional to convert between different forms of antibodies with their knowledge of molecular biology and bioinformatics.
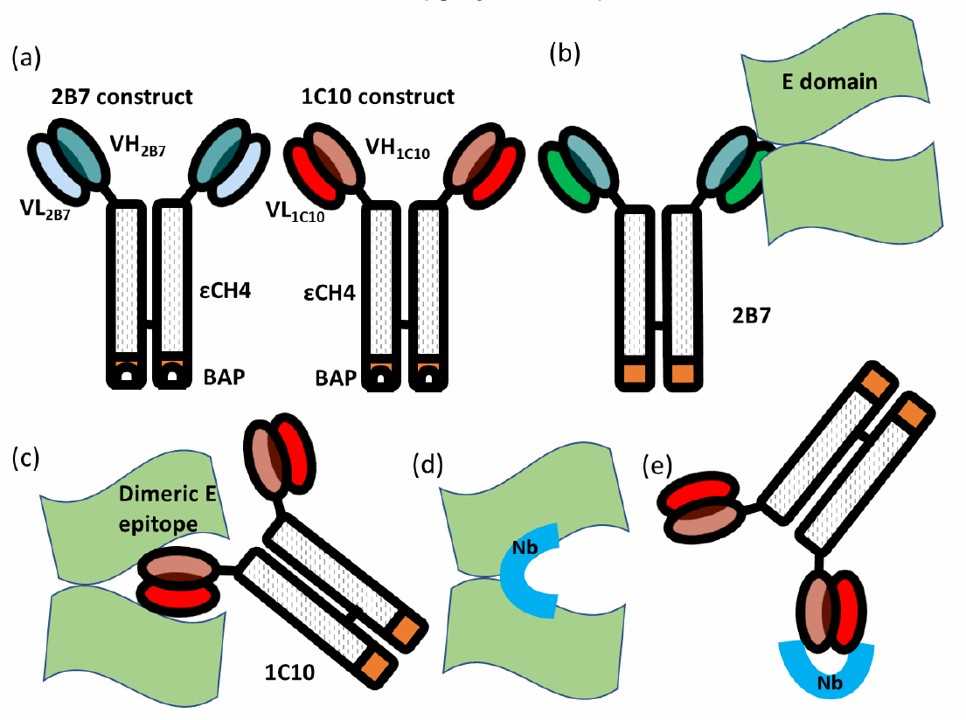 Fig. 1 Representation of the biological material used for anti-idiotypic selection. (Monica Poggianella, 2023)
Fig. 1 Representation of the biological material used for anti-idiotypic selection. (Monica Poggianella, 2023)
Single domain antibodies are derived from the blood of camelids (llama, alpaca, camel, dromedary, etc.). Different from conventional antibodies, the camelid "heavy-chain antibodies" (HCAbs) lack a light chain and are bound to antigen in the nm range with a single domain called VHH due to their small size. Like conventional antibodies, single domain antibodies show well-performed target specificity, high affinity, and low inherent toxicity. Like small molecule drugs, they are able to inhibit enzymes and readily access receptor clefts, which is comparable with conventional antibodies.
Please click here to see more custom anti-idiotypic antibody production services from Creative Biolabs.
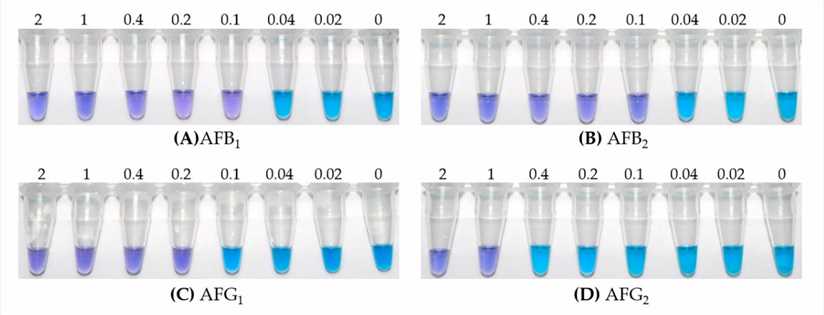 Fig. 2 Visual detection limits of iLAMP for four aflatoxins. (Jiawen Lei, 2020)
Fig. 2 Visual detection limits of iLAMP for four aflatoxins. (Jiawen Lei, 2020)
Aflatoxin is a highly toxic secondary metabolite produced by Aspergillus fungi and is classified as a class I carcinogen of liver cancer. Monitoring aflatoxin in all kinds of food and feed is an important means to protect public health. In this study, an immuno-loop-mediated isothermal amplification (iLAMP) assay for aflatoxin detection was developed based on anti-aflatoxin monoclonal antibody 1C11 (mAb 1C11) and anti-idiotypic single domain antibody-phage V2–5 specific for mAb 1C11. The new iLAMP detection does not need aflatoxin conjugates, an antibody labeling process, or special equipment. It provides an alternative to the existing methods and has the advantages of being time-saving, cost-effective, and easy to use.
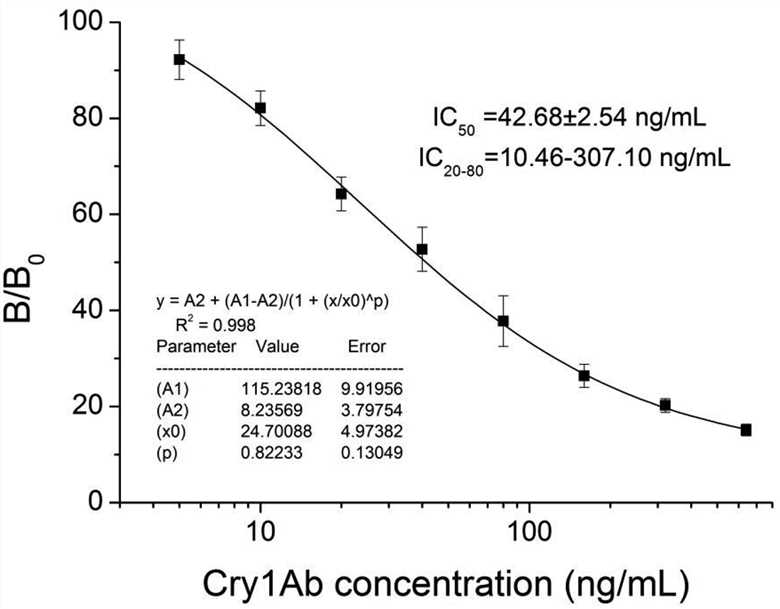 Fig. 3 Standard competitive inhibition curve for Cry1Ab analysis under the optimized conditions. (Yulou Qiu, 2020)
Fig. 3 Standard competitive inhibition curve for Cry1Ab analysis under the optimized conditions. (Yulou Qiu, 2020)
Cry toxins have been widely used in pest control of genetically modified organisms, which has aroused public concern about their impact on the natural environment and food safety. In this study, four anti-idiotypic single-domain antibodies specifically binding to the anti-Cry1Ab monoclonal antibody (MAb) were successfully selected from the initial phage display sdAb library established by camel peripheral blood lymphocytes. Subsequently, the researchers established a phage-mediated competitive chemiluminescence immunoassay (c-CLIA) based on anti-idiotypic sdAb for the determination of Cry1Ab. The results showed that the established method was highly specific for the recognition of Cry1Ab toxin, and the cross-reactivity with other Cry toxins was negligible. Using anti-idiotypic single-domain antibodies based on phage display as competitive antigen mimics, the proposed c-CLIA may provide an alternative strategy for Cry1Ab toxin analysis.
Anti-idiotypic single-domain antibodies are designed to specifically bind the antigen binding site of the antibody, which is a unique group of antigenic determinants (idiotopes) in the variable region of the antibody. Anti-idiotypic sdAb targets these unique sites and effectively mimics the structure of the original antigen recognized by antibodies. This makes them useful tools for studying antibody-antigen interactions and for therapeutic interventions, such as helping to regulate the immune response in vaccine development.
The first is the recognition and selection of epitopes of specific antibodies, which depends on an in-depth understanding of the structure and function of antibodies. Next, the single domain antibodies that can bind to these epitopes with high affinity were screened using phage display libraries, or yeast display libraries. The selected sdAb candidates will be further characterized, including their affinity, specificity, stability, and biological activity. After confirming that the candidate sdAbs has the expected characteristics, it is transferred to the production stage, which is usually expressed by bacteria such as E. coli. The production of sdAbs needs to ensure its purity and activity and finally carry out strict quality control tests to ensure the consistency and safety of each batch of products.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
