- Home
- ADC Development
- ADC In Vitro Analysis
- ADC Biochemical Analysis
- ADC Structure Analysis
ADC Structure Analysis Service
As a recognized industrial leader in antibody-drug conjugate (ADC) development, Creative biolabs has established a comprehensive analytical platform to evaluate the biochemical properties of a newly designed ADC. We are experienced and committed to provide our clients with top-quality services to elucidate the structural information of your ADC.
Antibody-drug conjugates are a new class of bio-therapeutic complexes that are produced by conjugating highly potent cytotoxic drugs to tumor targeting monoclonal antibodies via a meticulously designed chemical linker. Compared to traditional chemotherapeutic anti-cancer medications, ADCs have achieved targeted drug delivery that increases the total therapeutic efficacy and reduces drug cytotoxicity. The structure of an ADC is a collective combination of its individual components and the chemical conjugation process that leads to ADC generation adds great complexity and heterogeneity to the ADC structure. Combined with their high molecular weights, ADC structural analysis represents a major challenge for analytical scientists.
On a face value, antibody-drug conjugates are more prone for aggregation due to the hydrophobicity of the conjugated payloads. Therefore, the structure of each component (drug, linker and mAb) of an ADC must be redefined using the most appropriate analytical methods. This is not only beneficial for ADC designing, since the structural information of an ADC is a prerequisite for sophisticated downstream evaluations (stability, efficacy…), but also provides a guideline for ADC production.
Here at Creative Biolabs, we analyze the protein fragments/aggregates, charge status, primary structure, secondary/tertiary structures, and post translational modifications (mainly glycosylation) of an ADC to obtain a full picture of its structure:
- Fragments/Aggregates: the conjugation process might lead to certain degree of protein degradation and this is identified and evaluated using methods such as SDS-PAGE and size exclusion chromatography coupled multi-angle laser light scattering (SEC-MALS).
- Charge Status: conjugation to Lys residues will affect the total charge status of the resulted ADC and this characteristic is essential for ADC purification. We measure ADC charge properties using high-resolution ion exchange chromatography.
- Primary Structure: we determine the exact site of payload conjugation (see Conjugation Sites Analysis for more information) using advanced chromatographic approach in combination with tandem mass spectroscopy.
- Secondary/Tertiary Structure: we evaluate the higher ordered protein structure in an ADC using methods such as circular dichroism (CD) spectroscopy or cryo-electron microscopy to ensure the correct protein confirmation after conjugation.
- Post Translational Modifications (Glycosylation): conjugation chemistry might have impact on glycosylation and sometimes the conjugation is achieved via the glycan portion of a mAb. The oligosaccharides are released by enzymatic deglycosylation (NGase F) and the composition determined by methods such as monosaccharide composition analysis and methylation-based linkage analysis using LC-MS.
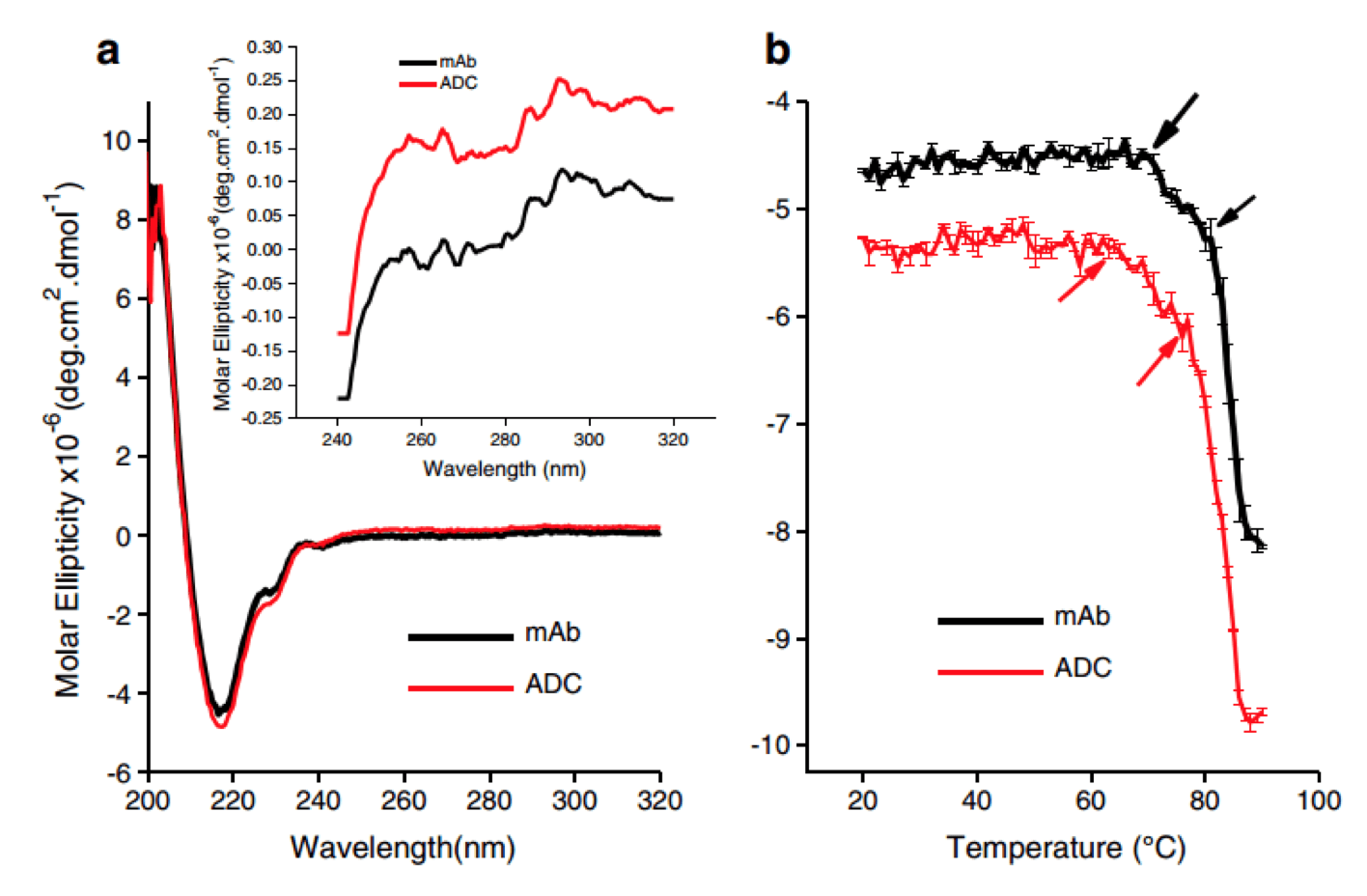 Circular dichroism measurements of an ADC compared to its mAb. The conjugation process exerted minor structural distortion to the mAb (Pharm Res., 2014)
Circular dichroism measurements of an ADC compared to its mAb. The conjugation process exerted minor structural distortion to the mAb (Pharm Res., 2014)
Creative biolabs has been devoted to help clients with the development of ADCs. We offer high-quality customized analytic services for ADC biochemical evaluation in a timely and cost efficient manner. Please contact us for more information and a detailed quote.
Reference:
- Guo, J.; et al. Assessment of physical stability of an antibody drug conjugate by higher order structure analysis: impact of thiol-maleimide chemistry. Pharm Res. 2014, 31(7): 1710-1723.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
