- Home
- ADC Development
- ADC In Vitro Analysis
- ADC In Vitro Efficacy Evaluation
- ADC In Vitro Cytotoxicity Evaluation
ADC In Vitro Cytotoxicity Evaluation Service
In vitro efficacy of an antibody-drug conjugate (ADC) is an important parameter in ADC development projects. It is the first qualitative evaluation of the drug efficacy of an ADC and it provides a baseline for ADC improvement and a guideline for downstream process designs. Using our advanced analytical platform, Creative biolabs provides our global clients with comprehensive ADC in vitro cytotoxicity analyses via the evaluation of cell viability, cell apoptosis, cell cycle status… using our large collection of tumor cell lines.
ADCs are a new class of targeted drugs with enhanced target specificity and drug efficacy. New generation of ADCs benefit from a series of improvements in payload potency, site-specific conjugation strategies, and innovated chemical linkers. ADCs exert excellent anti-tumor efficacies and in the meantime, an improved safety profile for normal tissues. Good ADC efficacy, both in vitro and in vivo, is the main focus in any ADC development projects since it is the ultimate goal of the pre-clinical studies and the prelude to clinical evaluations.
Prior to in vivo activity/potency evaluation, the in vitro efficacy of ADCs, especially their cytotoxicity, must be fully evaluated. In vitro cell cytotoxicity assay is widely used for this very purpose. In this set of assays, the ADC toxicity is tested against a variety of tumor cell lines expressing the targeted surface antigen. The in vitro efficacy of an ADC is directly reflected by cell viability analysis, in which the total number of the viable cells after ADC exposure is assessed by various organic dyes. In addition, other assays such as apoptosis and cell cycle assays are also used to analyze and confirm the subcellular targets and working mechanism of the ADC. Last but not least, when assessing the ADC in vitro efficacy, normal cells are also involved in this assay to evaluate the in vitro safety of the tested ADC.
Here at Creative biolabs, we perform various analyses to fully evaluate the in vitro cytotoxicity of your ADCs using this work-flow:
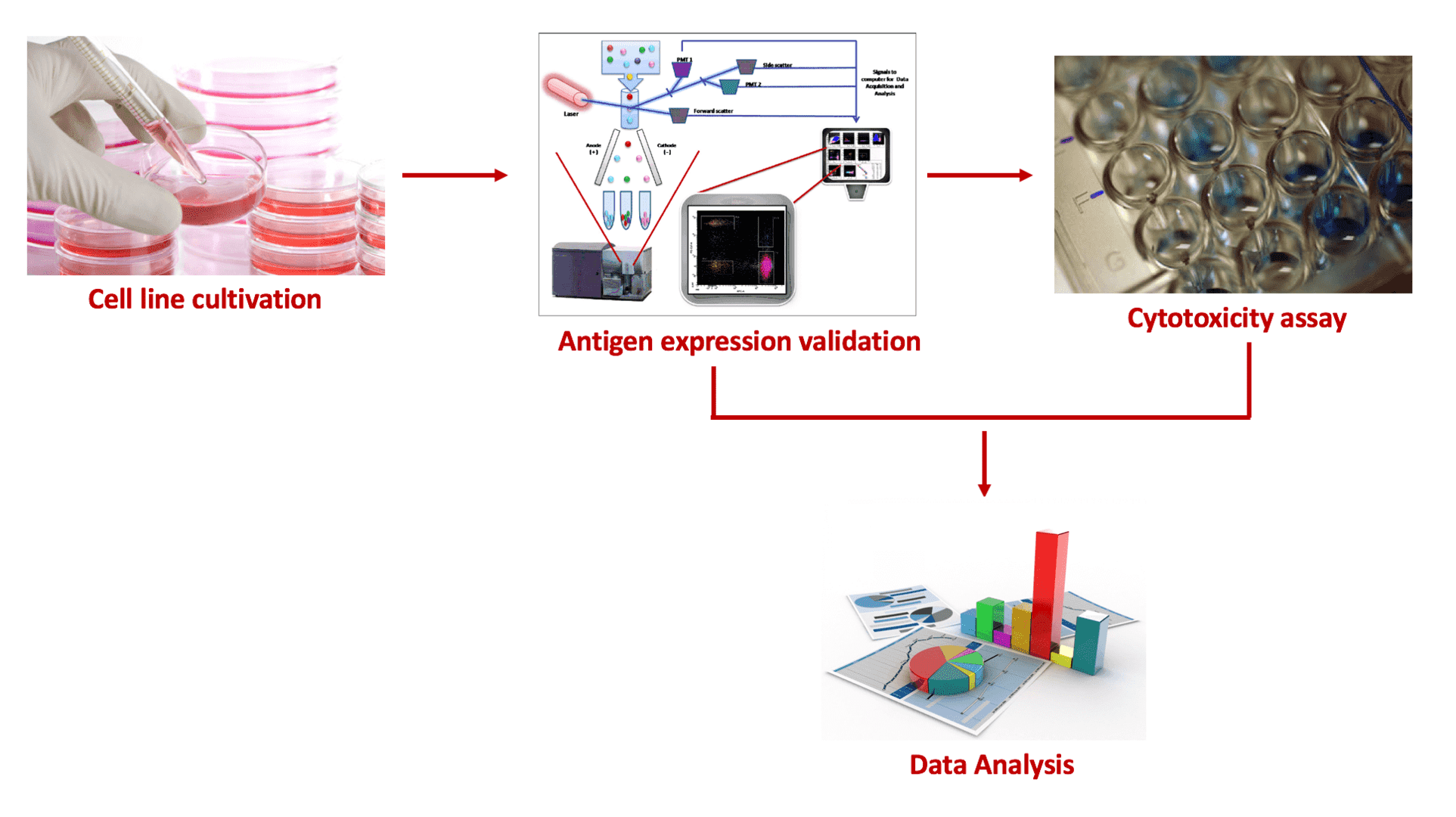 A work flow of ADC in vitro cytotoxicity assay. In the analysis both cancer cells expressing targeted antigens and normal cells are used for a comprehensive evaluation of the cytotoxicity and safety of an ADC, respectively.
A work flow of ADC in vitro cytotoxicity assay. In the analysis both cancer cells expressing targeted antigens and normal cells are used for a comprehensive evaluation of the cytotoxicity and safety of an ADC, respectively.
- Cell line cultivation—both cancer cells expressing the target antigen and normal cells are cultured for ADC toxicity analysis.
- Antigen expression validation—flow cytometry methods, such as Fluorescence-activated cell sorting (FACS) assay, are used to validate and quantify the surface expression of the targeted antigen and also to evaluate ADC binding to the antigen.
- Cytotoxicity assay—the ADC cytotoxicity is assessed using
- Cell viability assay—viable cells are detected by Presto Blue, CCK-8 or other reagents.
- Cell apoptosis measurement—cells undergo apoptosis are detected by Annexin V-FITC, propidium iodide staining, or other reagents.
- Cell cycle analysis—cells in different stages of cell cycle is detected by propidium iodide staining or other corresponding reagents.
- Data analysis—data gathered from these assays are compiled and analyzed using appropriate software packages.
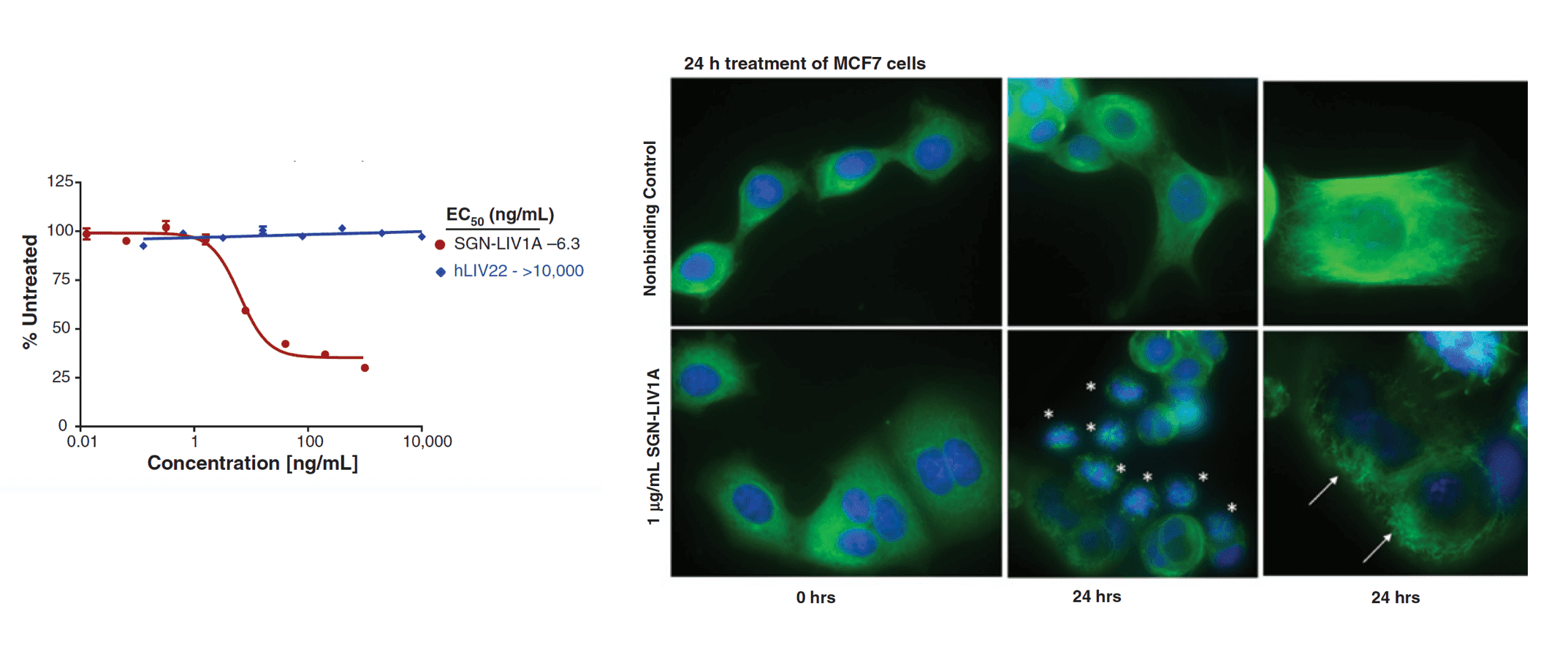 ADC in vitro cytotoxicity analysis. The in vitro cytotoxicity of an ADC is reflected by the EC50 value derived from cell viability assay (left) while the inhibitory effect of the ADC on microtubule assembly is visualized using fluorescent staining (right, Mol Cancer Ther, 2014).
ADC in vitro cytotoxicity analysis. The in vitro cytotoxicity of an ADC is reflected by the EC50 value derived from cell viability assay (left) while the inhibitory effect of the ADC on microtubule assembly is visualized using fluorescent staining (right, Mol Cancer Ther, 2014).
Creative biolabs is a leader in antibody-drug conjugate developments with extensive experience in antibody discoveries, antibody productions, and synthetic organic chemistry. We offer comprehensive analytical platform for a full characterization of your customized ADCs. Please contact us for more information and a detailed quote.
Reference:
- Sussman, D.; et al. SGN–LIV1A: a novel antibody–drug conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Mol Cancer Ther. 2014, 13(12): 2991–3000.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
