- Home
- ADC Development
- ADC In Vitro Analysis
- ADC Biochemical Analysis
- ADC Stability Analysis
ADC Stability Analysis Services
Creative Biolabs offers highly customized service packages for antibody-drug conjugates (ADCs) characterization. With advanced analytical platforms and experienced lab personnel, we perform top-quality services to address the stability profile of an ADC, including ADC physical stability, chemical stability, and in vitro /in vivo plasma stability.
ADCs are a class of therapeutic agents that are generated via the conjugation of cytotoxic small molecule drugs to cancer targeting monoclonal antibodies through a chemical linker. By concept, an ADC delivers the toxic drug specifically to the cancer site and releases it within cancer cells. To fulfill this design, an ADC must first exert good stability under different physiological pH buffer systems without releasing of the payload drug (chemical stability) and then show stability within systemic circulation to prevent off-target drug release (plasma stability). In the meantime, an ADC should also exert good physical stability to ensure decent solubility and avoid aggregation/precipitation….
Both ADC physical and chemical stabilities are important factors in ADC formulation and development. On the other hand, the plasma stability of an ADC is critical for its performance since it determines the off-target drug release in circulation and has profound impact on the safety and therapeutic efficacy of an ADC. Thus, for a newly generated ADC complex, its stability must be carefully validated before its deployment in animal or human systems.
Creative Biolabs is in possession of a series of sophisticated laboratory equipment for ADC stability analysis, including:
- UV-Vis spectroscopy
- Intrinsic and extrinsic fluorescence spectroscopy
- Differential scanning calorimetry (DSC)
- Circular dichroism (CD) spectroscopy
- Dynamic light scattering
- Capillary isoelectric focusing (cIEF)
- Imaged capillary SDS-PAGE
- Size-exclusion chromatography (SEC) with UV or MALS detection
- Hydrophobic interaction chromatography (HIC)
- ELISA Assays
- Reverse phase HPLC (RP-HPLC)
- Liquid chromatography-mass spectrometry (LC-MS)
- Surface plasmon resonance (SPR)
- Plasma incubation, affinity capture of ADC, HIC, and LC-MS
Using our advanced technological platform, Creative Biolabs provides a broad-spectrum ADC stability analysis services, including:
- ADC Physical stability
- Thermal stability assessment
- Photic stability assessment
- ADC Chemical stability
- Chemical stability of the antibody
- Chemical stability of the payloads and linkers (Payload release)
- Chemical instability related to the conjugation process
- ADC Plasma stability
- In vitro plasma stability
- In vivo plasma stability
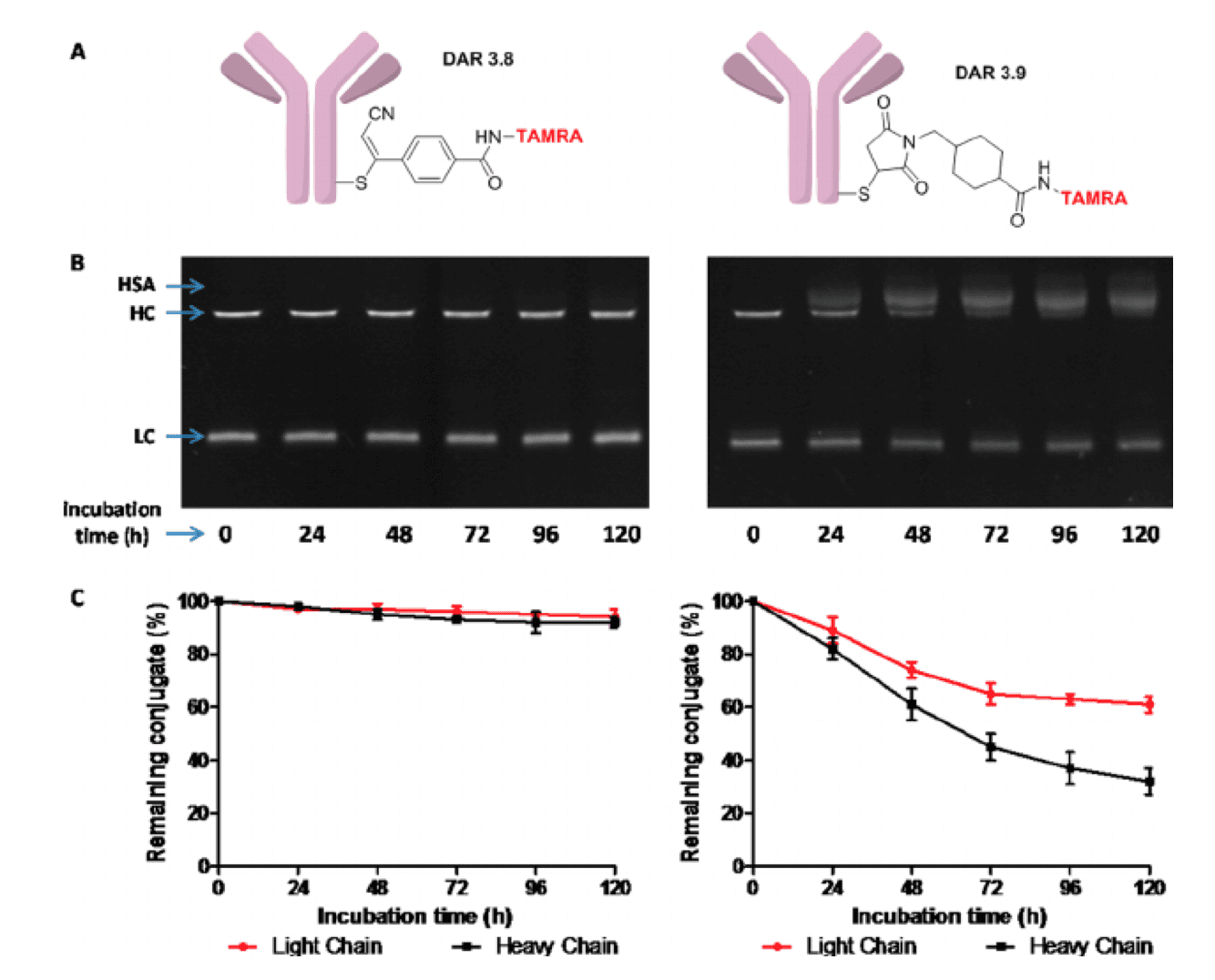 Comparison of plasma stability of CBTF- and SMCC-based conjugates. The CBTF conjugate exerted better plasma stability than the SMCC conjugated ADC (Bioconjugate Chem., 2015).
Comparison of plasma stability of CBTF- and SMCC-based conjugates. The CBTF conjugate exerted better plasma stability than the SMCC conjugated ADC (Bioconjugate Chem., 2015).
With our experienced science team and advanced analytical platform, Creative Biolabs is dedicated to provide our global clients with the comprehensive analytical services for ADC development projects. Please contact us for more information and a detailed quote.
References:
- Kolodych, S.; et al. CBTF: New amine-to-thiol coupling reagent for preparation of antibody conjugates with increased plasma stability. Bioconjugate Chem. 2015, 26(2): 197-200.
- Ross, P.L.; et al. Physical and chemical stability of antibody drug conjugates: current status. J. Pharm. Sci. 2016, 105: 391-397.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
