- Home
- ADC Development
- ADC In Vitro Analysis
- ADC Biochemical Analysis
- DAR & Payload Distribution Analysis
DAR and Payload Distribution Analysis Service
Creative Biolabs is a leader and explorer in novel therapeutics development. With our Ph.D. level scientists and extensive experience in antibody-drug conjugate (ADC) chemistry, Creative Biolabs now provides our customers with elaborated analysis services for the complete assessment of the drug to antibody ratio (DAR) and drug load distribution of an ADC.
Antibody-drug conjugate is a unique class of promising therapeutics agents, in which one or several cytotoxic drug molecules are covalently linked to a monoclonal antibody that specifically binds to an antigen expressed on the surface of tumor cells to achieve targeted drug delivery. Many unique parameters characterize an ADC and determine its efficacy, including ADC structure, stability, drug to antibody ratio (DAR), payload distribution….
DAR is one of the most important quality parameters of an ADC and it is presented as the average number of drug molecules conjugated to an antibody. Payload distribution, determined by fractionation of antibodies containing different number of drugs, is another quality attribute of an ADC. DAR and payload distribution are not only the measurements of ADC product homogeneity but they also determine the amount of payload delivered to the target tissues, directly affecting both ADC efficacy and safety. What’s more, DAR and payload distribution assessments are both important quality control criteria in ADC manufacturing.
Various analytical approaches for DAR and payload distribution assessment are available from Creative Biolabs:
Ultraviolet-Visible (UV/Vis) spectroscopy
UV/Vis spectroscopy is the simplest and most convenient approach for DAR determination and works well with a wide range of conjugation methods, including cysteine-linked or lysine-linked ADCs. This method only requires that the UV/Vis spectrum of the antibody and the payload have different maximum absorbance wavelengths (Amax). Upon measuring the extinction coefficients of the antibody at 280 nm and that of the drug at its Amax, the individual concentrations of mAb and drug can be determined by the solution of two simultaneous equations, from which the molar ratio (moles of drug per mole of antibody) can be calculated. The DARs of several ADCs have determined using this technique, including immuno-conjugates bearing doxorubicin, calicheamicin analogs, maytansinoid, and monomethyl auristatin.
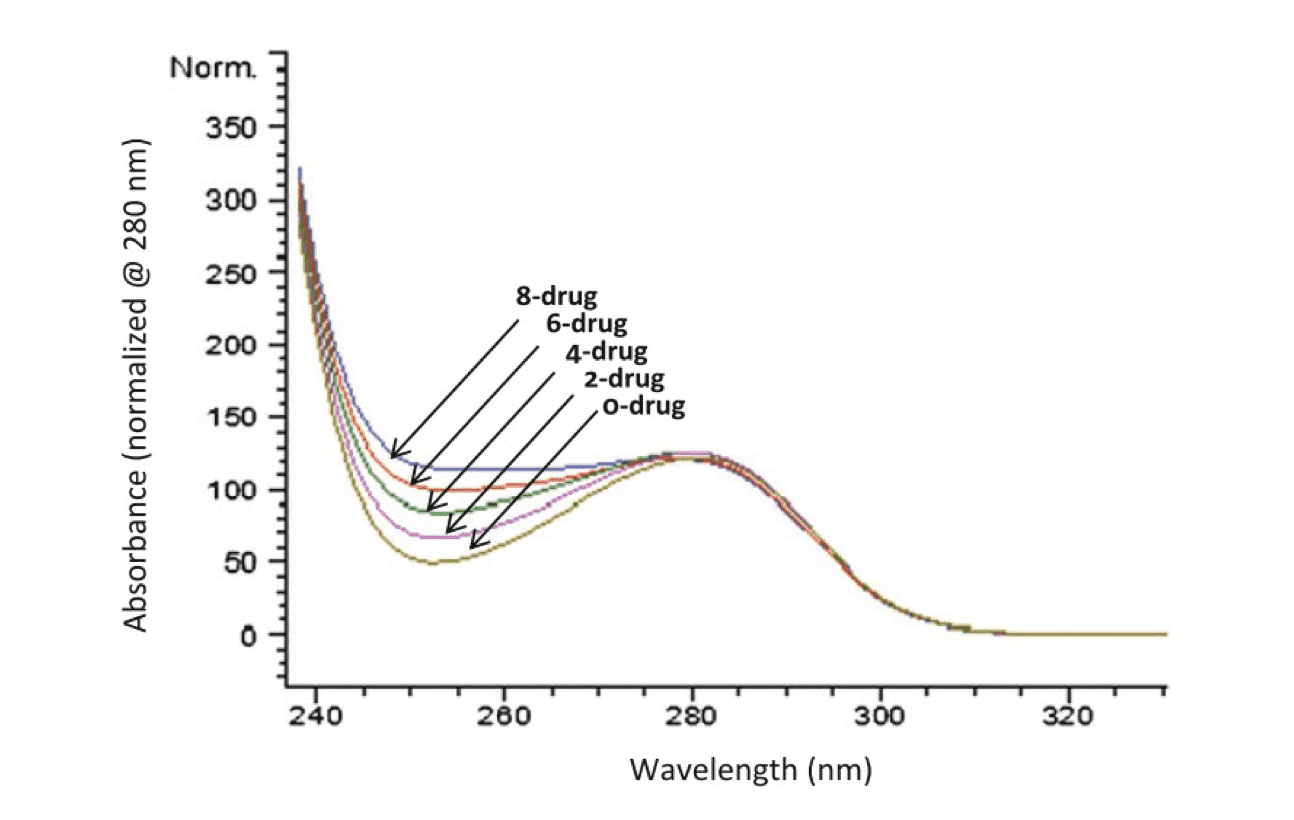 UV spectra of drug-loaded species. An overly of the UV spectra of all the ADC species, normalized to 280 nm absorbance, showing an increase in 248 nm absorbance as the number of conjugated drug increases (Antibody-drug conjugates., 2013).
UV spectra of drug-loaded species. An overly of the UV spectra of all the ADC species, normalized to 280 nm absorbance, showing an increase in 248 nm absorbance as the number of conjugated drug increases (Antibody-drug conjugates., 2013).
Hydrophobic interaction chromatography (HIC)
HIC is a leading technique for the characterization of DAR values and drug loading distribution. It is often used to characterize cysteine-conjugated ADCs. The conjugated species are separated based on an increased hydrophobicity caused by the increased drug-load. In terms of cysteine-conjugated ADCs, the unconjugated antibody with the least hydrophobicity is eluted first while the most hydrophobic, 8-drug conjugated form elutes last, generating a quantitative elution profile. The area percentage of a peak represents the relative amount of each drug-loaded ADC species. The payload distribution is derived from the HIC profile while the average DAR is also calculated from the percentage peak area.
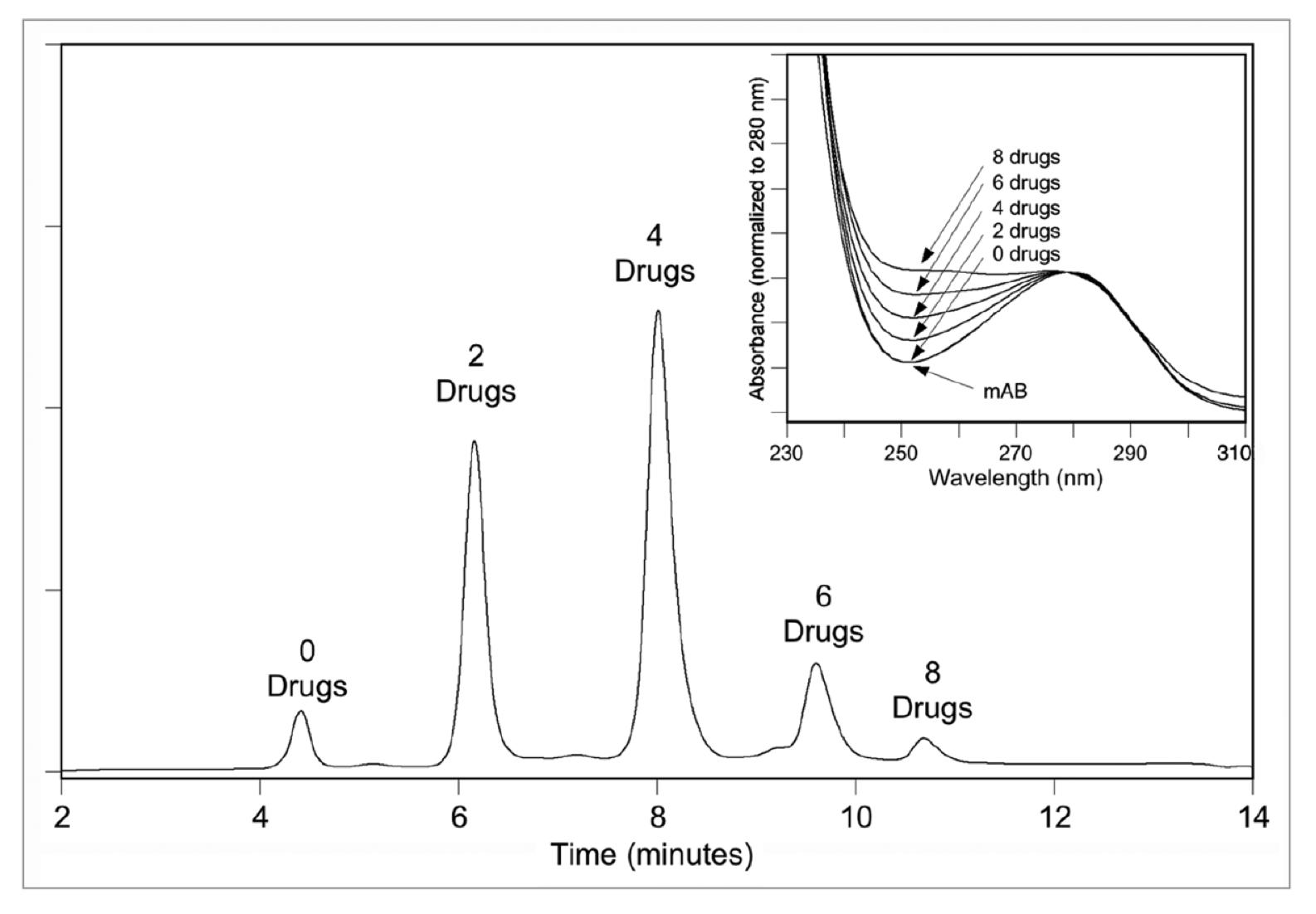 A representative HIC chromatogram. HIC profile of an ADC (mAb-vc-MMAE), yielding five peaks that correspond to drug-load species with zero, two, four, six and eight drugs (mAbs., 2011).
A representative HIC chromatogram. HIC profile of an ADC (mAb-vc-MMAE), yielding five peaks that correspond to drug-load species with zero, two, four, six and eight drugs (mAbs., 2011).
Reversed phase high-performance liquid chromatography (RP-HPLC)
RP-HPLC is another detection technique based on hydrophobicity that suits DAR measurement for Cys-linked ADCs. Using this method, the light and heavy chains of an ADC are first dissociated by a reducing agent, followed by the separation using a reverse-phase column. The percentage peak area from the integrated light chain and heavy chain peaks, combined with the assigned drug load for each peak, is used to calculate the weighted average DAR.
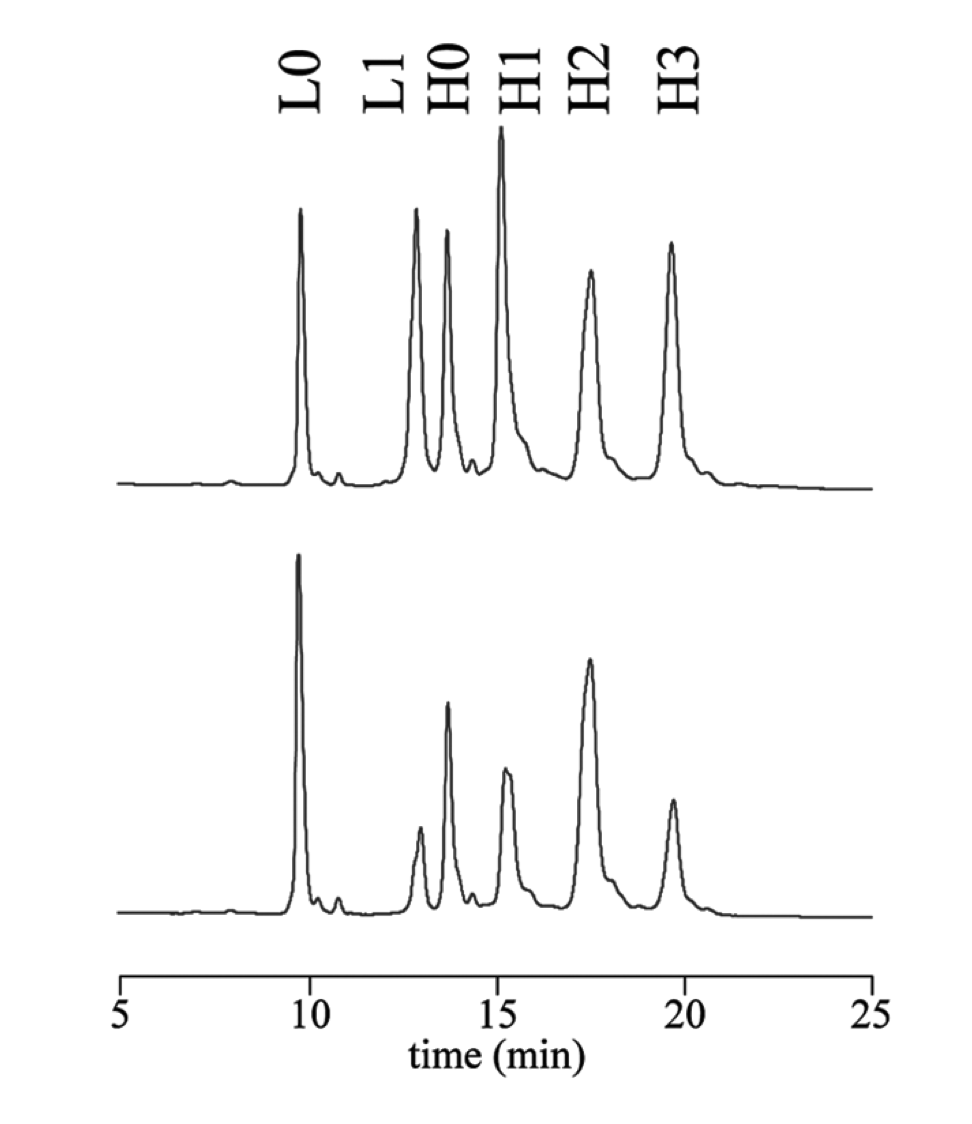 A representative RP-HPLC chromatogram (mAbs., 2011).
A representative RP-HPLC chromatogram (mAbs., 2011).
Liquid chromatography coupled with electrospray ionization masss pectrometry (LC-ESI-MS)
LC-ESI-MS is an orthogonal technique comparing to UV/Vis spectroscopy, HIC, and RP-HPLC in DAR and payload distribution assessment. It depends on both the chemistry of the linker-drug and the method of conjugation (e.g., cysteine-linked or lysine-linked). While HIC and RP-HPLC techniques are limited to ADCs with a narrow number of conjugation sites (e.g., at inter-chain cysteines or engineered cysteines), LC-ESI-MS is in practice suitable for the analysis of more heterogeneous ADCs such as the ones generated via lysine conjugation. Since lysine-linked ADCs are stable and remain intact under denaturing conditions, the different drug-loaded forms of the lysine-linked ADCs can be accurately identified by their masses. However, due to their high heterogeneity, the interpretation of the mass spectrum of lysine-linked ADCs can be challenging. Therefore, additional sample preparation methods such as the removal of C-terminal lysine heterogeneity and deglycosylation of the ADC are often needed to reduce the spectral complexity. Finally, the ADCs are desalted using a reversed-phase LC column, and analyzed by MS. The MS spectrum is processed (de-convoluted) and converted into a series of zero charge state masses that correspond to the increased number of drugs in the conjugates.
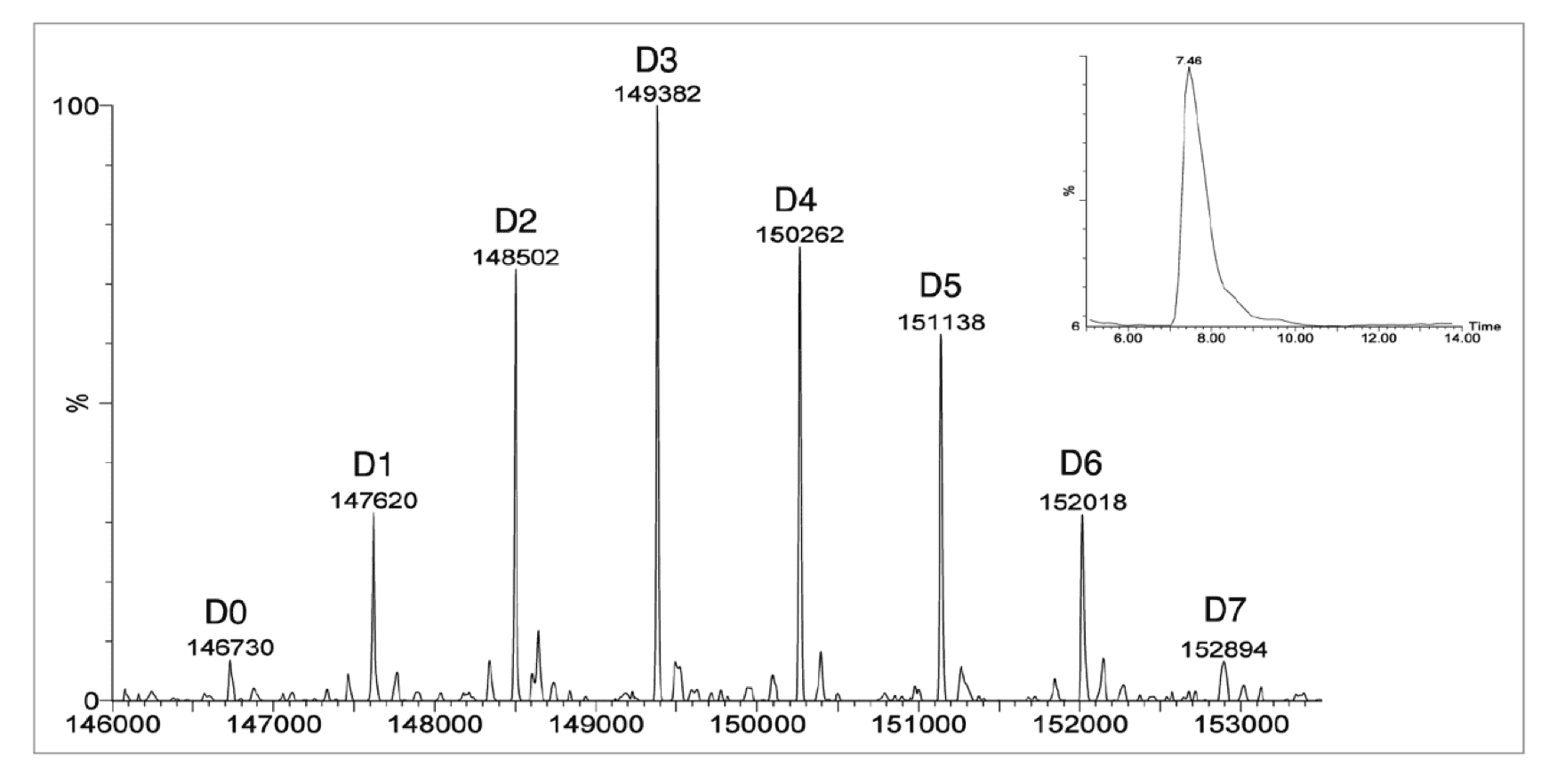 Deconvoluted mass spectra of a lysine-linked ADC. The observed masses correspond to the antibody (Ab) with drug load (n) of 0–7 (mAbs., 2011).
Deconvoluted mass spectra of a lysine-linked ADC. The observed masses correspond to the antibody (Ab) with drug load (n) of 0–7 (mAbs., 2011).
Creative Biolabs has years of experience in antibody discoveries and productions, chemical synthesis, as well as conjugate analysis. We are dedicated to offer our clients with top-quality DAR and drug load distribution assessment to meet your project requirements. Please contact us for more information and a detailed quote.
Reference:
- Wakankar, A.; et al. Analytical methods for physicochemical characterization of antibody drug conjugates. mAbs. 2011, 3(2): 161-172.
- Ducry, L.; et al. Antibody-drug conjugates. Methods in Molecular Biology. 2013, 1045.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
