All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
In the rapidly advancing fields of gene therapy, cancer treatment, and other biotechnological applications, plasmid DNA is a cornerstone for further development. Creative Biolabs is committed to providing comprehensive global CDMO services for plasmids ranging from research-grade to GMP-grade plasmid DNA, ensuring that every stage of drug development and production is covered efficiently and meticulously.
Creative Biolabs offers comprehensive Contract Development and Manufacturing Organization (CDMO) services for CART plasmids, which play a pivotal role in the transformative field of cell therapy. Our services span research-grade to GMP-grade production, tailored to meet an array of research, pre-clinical, clinical, and commercial needs.
Research Grade (RG) CAR-T Plasmid DNA Manufacturing
With our dedicated research-grade plasmid production, we deliver high-quality plasmid DNA suitable for basic research and early drug discovery processes. Utilizing stringent quality control (QC) measures, our research-grade plasmids are produced to support exploration and validation studies in the initial stages of CART development.
GMP-like CAR-T Plasmid Manufacturing
For pre-clinical studies, including animal testing of drug safety and metabolism, our GMP-like plasmid production adopts key features of Good Manufacturing Practices (GMP). This includes segregated production suites, document control, traceability, and optional antibiotic-free conditions, embodying a cost-effective mimic of full GMP processes.
GMP CAR-T Plasmid DNA Manufacturing
Our GMP plasmid DNA manufacturing service meets the highest quality standards, suitable for clinical and commercial applications. Produced in certified GMP suites, our plasmids adhere to rigorous quality assurance protocols encompassing a comprehensive array of in-process and release QC assays. Each batch comes with a detailed release report and certificate of analysis.
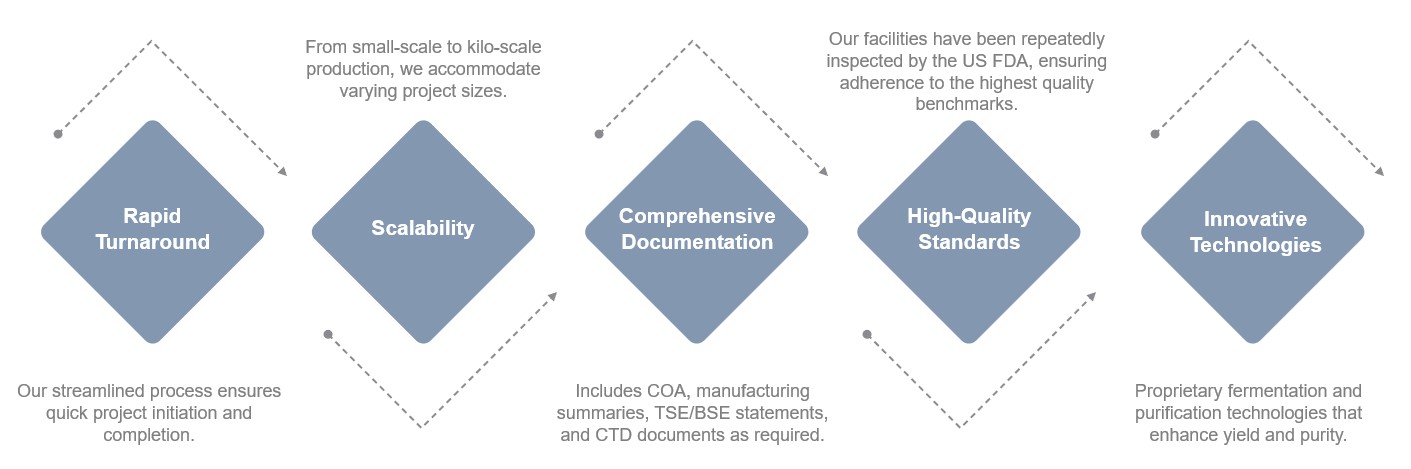
Creative Biolabs excels at catering to diverse production needs. Our small-scale service line offers rapid production of plasmid DNA, ideal for the transfer and packaging of plasmids, as well as mRNA manufacturing starting materials. For larger needs, we provide scalable GMP-compliant production, with fermentation capacities.
We support the intricate needs of advanced therapies, including DNA adjuvants, vaccines, and viral vector manufacturing (AAV and LVV). Our expansive capabilities encompass both clinical and commercial pDNA products, produced under rigorous GMP conditions.
Our expertise extends to non-viral DNA vector engineering and manufacturing. Leveraging our robust production process, we achieve high-quality plasmid DNA suitable for therapeutic applications, including sequences with complex elements like ITRs, LTRs, and polyA tails.
Detailed analysis and optimization of the plasmid vector.
Scalable fermentation and plasmid extraction.
Establishment of robust cultures and cell banks.
Comprehensive QC assays and documentation.
At Creative Biolabs, we employ a stringent QC framework to ensure the highest quality of plasmid DNA. Key assays include:
Assessment of appearance, concentration, pH, osmolality, and aggregation.
Restriction enzyme digestion and sequencing.
Sterility tests and endotoxin determination.
Long-term and stress condition stability studies per ICH guidelines.
OD260/280 ratios, supercoiled plasmid determination through agarose gel electrophoresis and HPLC, residual protein analysis, and host DNA/RNA quantification.
Q1: What are the typical applications of your plasmid DNA products?
A1: Our plasmid DNA products are utilized in a variety of applications, including basic research, drug discovery, preclinical testing, clinical trials, and commercial biomanufacturing processes for CGT therapies.
Q2: Can you accommodate custom plasmid DNA production requirements?
A2: Yes, our flexible manufacturing capabilities allow us to customize plasmid DNA production to meet specific project needs, including special fermentation conditions and customized QC testing.
By choosing Creative Biolabs for your plasmid DNA needs, you are partnering with a leader in genetic medicine manufacturing, equipped to deliver high-quality plasmid DNA tailored to your specific research, preclinical, and clinical requirements.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION