All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Chimeric Antigen Receptor T-cell (CART) therapy represents a groundbreaking advancement in cancer immunotherapy. At the core of this therapeutic innovation lies the genetic engineering of T-cells using plasmid DNA vectors. The manufacturing of Good Manufacturing Practice (GMP)-like plasmid DNA is crucial to ensure the safety, efficacy, and regulatory compliance of these therapies. Creative Biolabs, with over two decades of industry experience, excels in providing superior GMP-like plasmid manufacturing services to support CART development.
GMP-like plasmid DNA is designed for pre-clinical studies such as animal testing of drug safety and metabolism. This grade adopts key features of GMP guidelines, ensuring a production process with stringent document control and traceability. Creative Biolabs leverages an advanced, streamlined manufacturing process to produce high-quality plasmid DNA for CART applications. Our facility is equipped with state-of-the-art technologies and adheres to rigorous GMP-like standards to ensure that each batch of plasmid DNA meets stringent quality and safety criteria.
Creative Biolabs provides a comprehensive spectrum of services to support every aspect of plasmid DNA manufacturing:
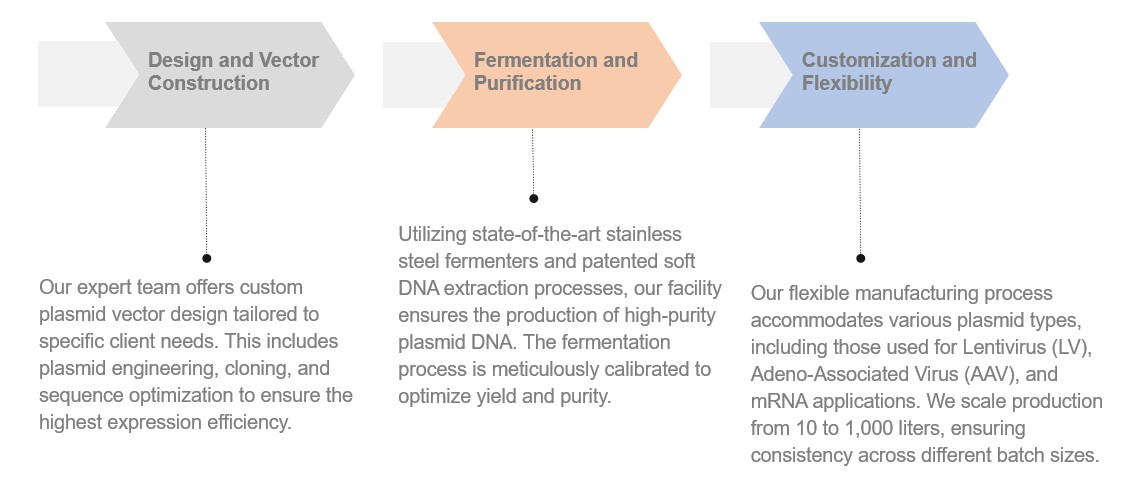
Our GMP-like plasmid manufacturing follows stringent quality assurance protocols to guarantee the safety and efficacy of the final product. Our quality control assays encompass a range of tests to ensure comprehensive quality assessment:
We provide detailed documentation for each production batch, including Certificates of Analysis (CoA), batch records, and traceability reports. These documents ensure full transparency and compliance with regulatory requirements.
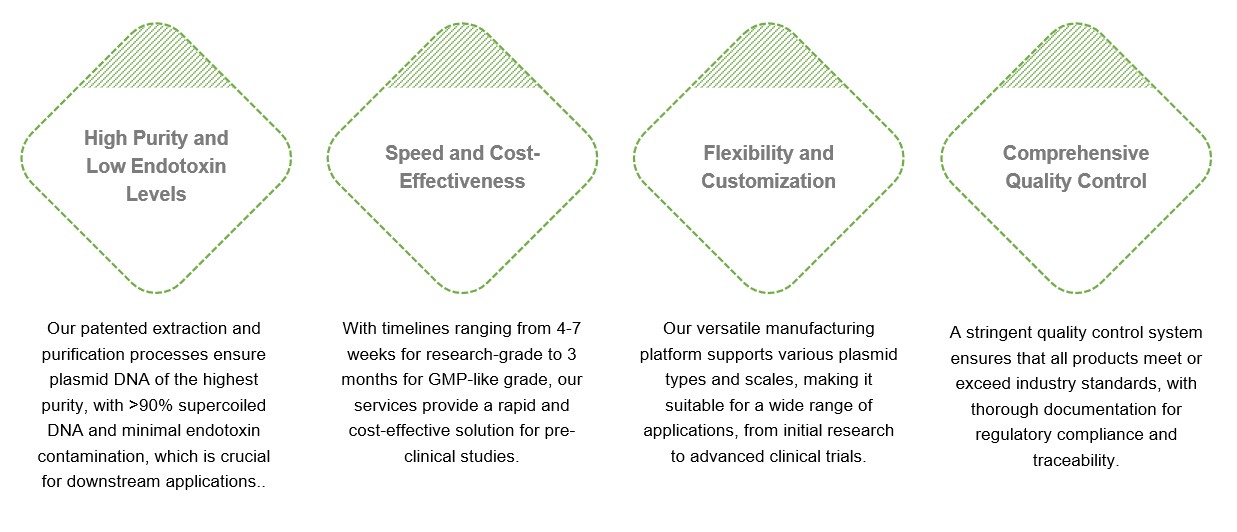
Creative Biolabs' GMP-like plasmid manufacturing service offers several unique advantages:
Q1: How does GMP-like manufacturing differ from full GMP?
A1: GMP-like manufacturing incorporates key GMP features such as document control and traceability but is designed to be a more cost-effective and faster alternative for pre-clinical studies. The quality attributes are comparable, but the scale and some production specifics might differ.
Q2: What are the endotoxin levels in GMP-like plasmid DNA?
A2: Our GMP-like plasmid DNA has extremely low endotoxin levels, which is critical for maintaining high transfection efficiency and viability in cell lines.
By offering high-quality plasmids produced under meticulous conditions, we empower researchers and clinicians to advance in their efforts to develop innovative and life-saving treatments. Explore the full potential of our GMP-like plasmid manufacturing services and take your CART research and clinical applications to new heights.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION