All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Natural killer (NK) cells, critical components of the innate immune system, exhibit cytotoxic activity against infected or abnormal cells, including cancer cells and virus-infected cells. The heterogeneity in surface marker expression and functional properties among NK cell subsets has led to the development of diverse NK cell products. Incorporating CAR (chimeric antigen receptor) technology into NK cells enhances their targeting specificity and anti-tumor efficacy, presenting a novel strategy for cancer immunotherapy. CAR-NK cell therapy signifies an innovative frontier in cancer immunotherapy, leveraging the innate cytotoxicity of NK cells and the precision of CARs to selectively target and eradicate tumor cells.
CAR-NK cell therapy integrates genetic engineering, immune recognition, and targeted cytotoxicity to enhance the anti-tumor activity of natural killer cells.
Genetic engineering of NK cells
The CAR-NK vector product manufacturing process initiates with the isolation of natural killer cells from peripheral blood or umbilical cord blood. Subsequently, these NK cells undergo genetic modification to introduce and express a chimeric antigen receptor on their cell surface. CAR design typically integrates an extracellular antigen-recognition domain, often a single-chain variable fragment (scFv) targeting a specific tumor-associated antigen (TAA), coupled with intracellular signaling domains such as CD3ζ and co-stimulatory domains like CD28 or 4-1BB. This modification is accomplished through utilizing viral vectors (e.g., lentivirus or retrovirus) or non-viral methodologies (e.g., electroporation) to deliver and integrate the CAR gene into the NK cells, and subsequently produce CAR-NK vector products primed for targeted tumor cell recognition and cytotoxic activity.
Recognition and targeting
CAR-NK cells are engineered to recognize specific tumor-associated antigens (TAAs) expressed on the surface of tumor cells, and upon encountering the target antigen, the CAR on NK cells binds to the antigen, triggering intracellular signaling pathways.
Chimeric antigen receptor natural killer can recognize and target cancer cells through a specific antigen-recognition mechanism.
Upon encountering cancer cells, the scFv domain of the CAR on CAR-NK cells binds specifically to the targeted TAA, initiating intracellular signaling pathways through the CAR's intracellular domains, which commonly include CD3ζ and co-stimulatory domains like CD28 or 4-1BB. This signaling cascade activates the CAR-NK cell, leading to downstream cellular responses that finish up with in the destruction of the target cancer cell.
Activated CAR-NK cells exert cytotoxic effects on cancer cells by releasing cytotoxic granules containing perforin and granzyme, inducing apoptosis (programmed cell death) in the cancer cells. Additionally, CAR-NK cells can secrete cytokines such as interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), enhancing the anti-tumor immune responses.
The specificity of CAR-NK cell targeting is dictated by the antigen-binding specificity of the CAR, enabling CAR-NK cells to selectively recognize and eliminate cancer cells expressing the targeted TAA while sparing normal cells that do not express the TAA. This targeted recognition and cytotoxic activity highlight the potential therapeutic efficacy of CAR-NK cell immunotherapy for cancer treatment.
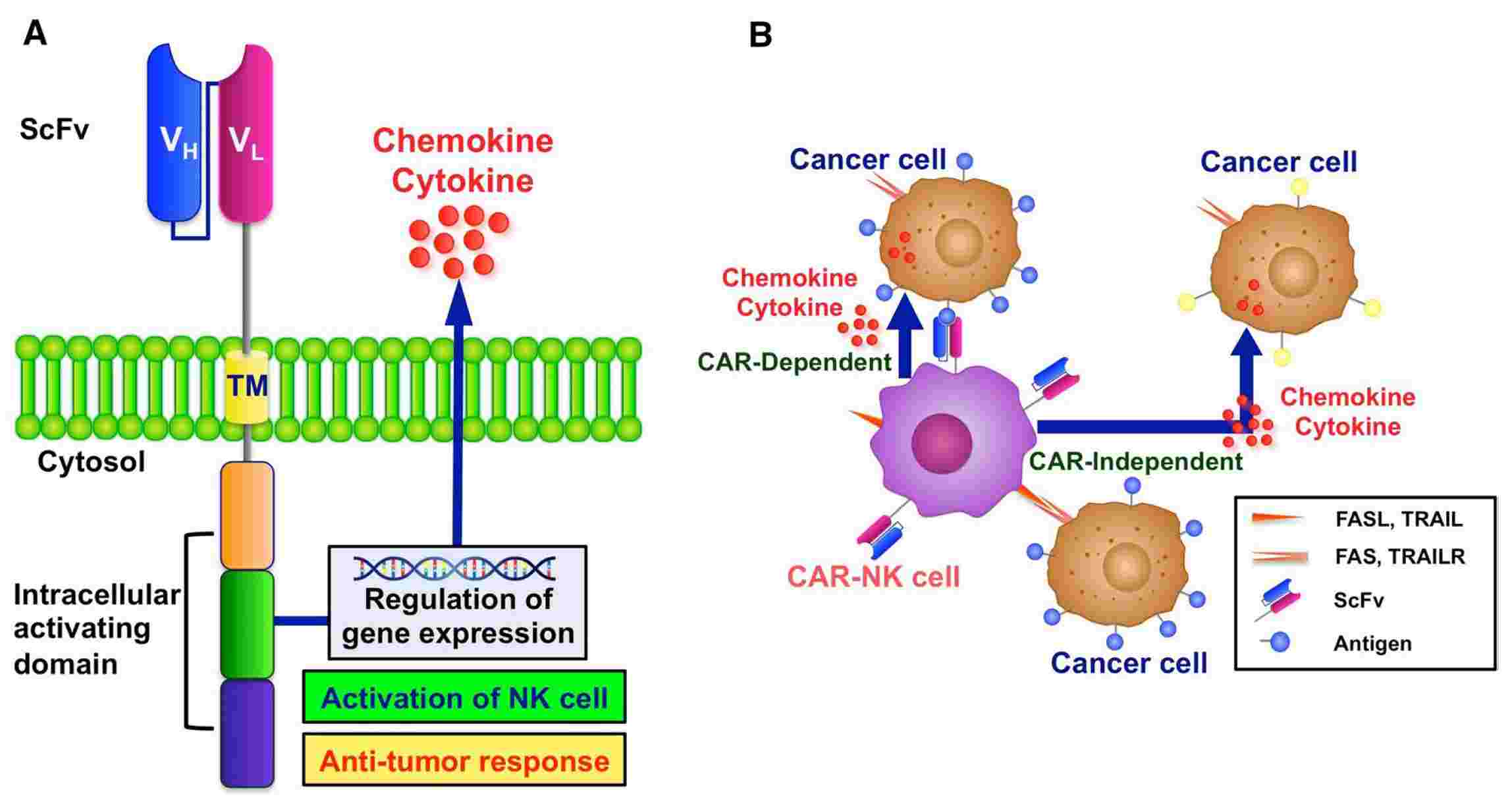 Fig 1. Structure of CAR-NK cells and the manner of tumor cell elimination. (Hamed,2022)
Fig 1. Structure of CAR-NK cells and the manner of tumor cell elimination. (Hamed,2022)
HER2-targeted CAR-NK cells in solid tumors
HER2-targeted CAR-NK therapies have been investigated in patients with HER2-positive solid tumors, including breast cancer and gastric cancer.
HER2 (human epidermal growth factor receptor 2), a cell surface receptor, is frequently overexpressed in various malignancies, particularly in breast and gastric cancers. This aberrant expression is often linked with aggressive tumor behavior and a dismal prognosis, rendering HER2 an strategic target for immunotherapeutic interventions. HER2-targeted CAR-NK cells are genetically modified to express CARs specifically recognize and bind to HER2-expressing tumor cells. Upon engagement with HER2, CAR-NK cells are activated, initiating targeted cytotoxicity and the subsequent destruction of HER2-positive cancer cells.
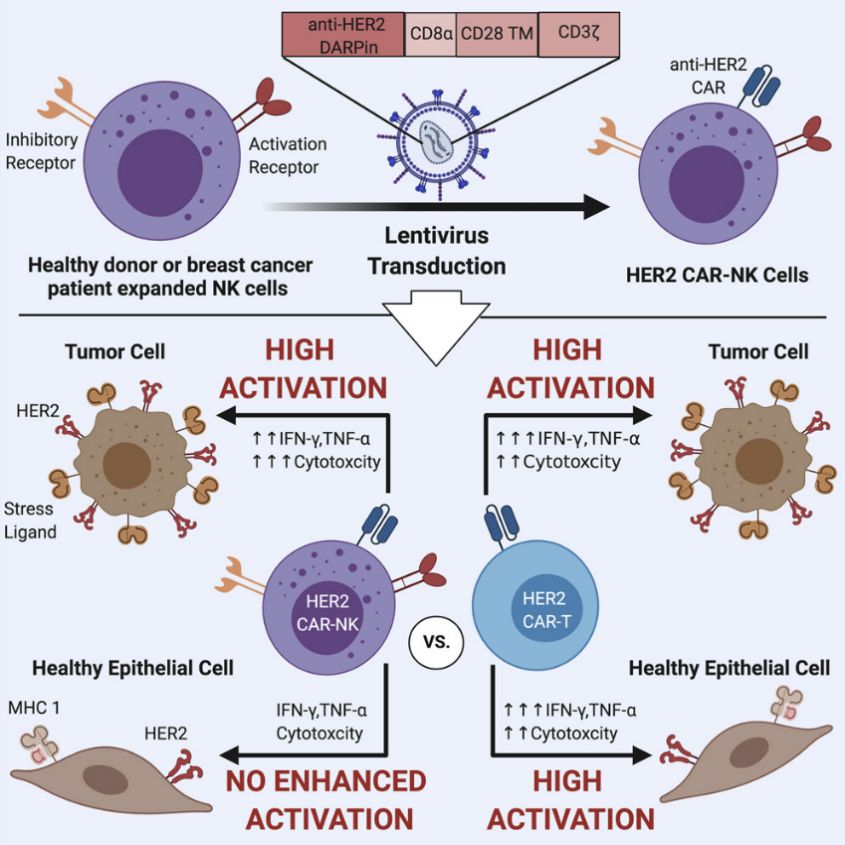 Fig 2. HER2-Targeted CAR-NK Cells in Solid Tumor (Ana, 2021)
Fig 2. HER2-Targeted CAR-NK Cells in Solid Tumor (Ana, 2021)
EGFR-targeted CAR-NK cells in solid tumors
EGFR is a transmembrane glycoprotein involved in regulating cell growth and survival, and its dysregulation is a hallmark of several solid tumors. EGFR-targeted CAR-NK cells are genetically engineered to express chimeric antigen receptors specifically recognize and bind EGFR on cancer cells. The CAR construct typically comprises an extracellular antigen-binding domain, such as a single-chain variable fragment (scFv), that selectively targets EGFR, coupled with intracellular signaling domains that activate natural killer cell effector functions upon engagement with the target antigen.
Preliminary studies in preclinical models have demonstrated the potential of EGFR-targeted CAR-NK cell therapy for addressing EGFR-driven malignancies. Further translational research is warranted to evaluate the safety, efficacy, and feasibility of this approach in clinical settings, with the ultimate goal of advancing personalized immunotherapeutic interventions for patients with EGFR-positive solid tumors.
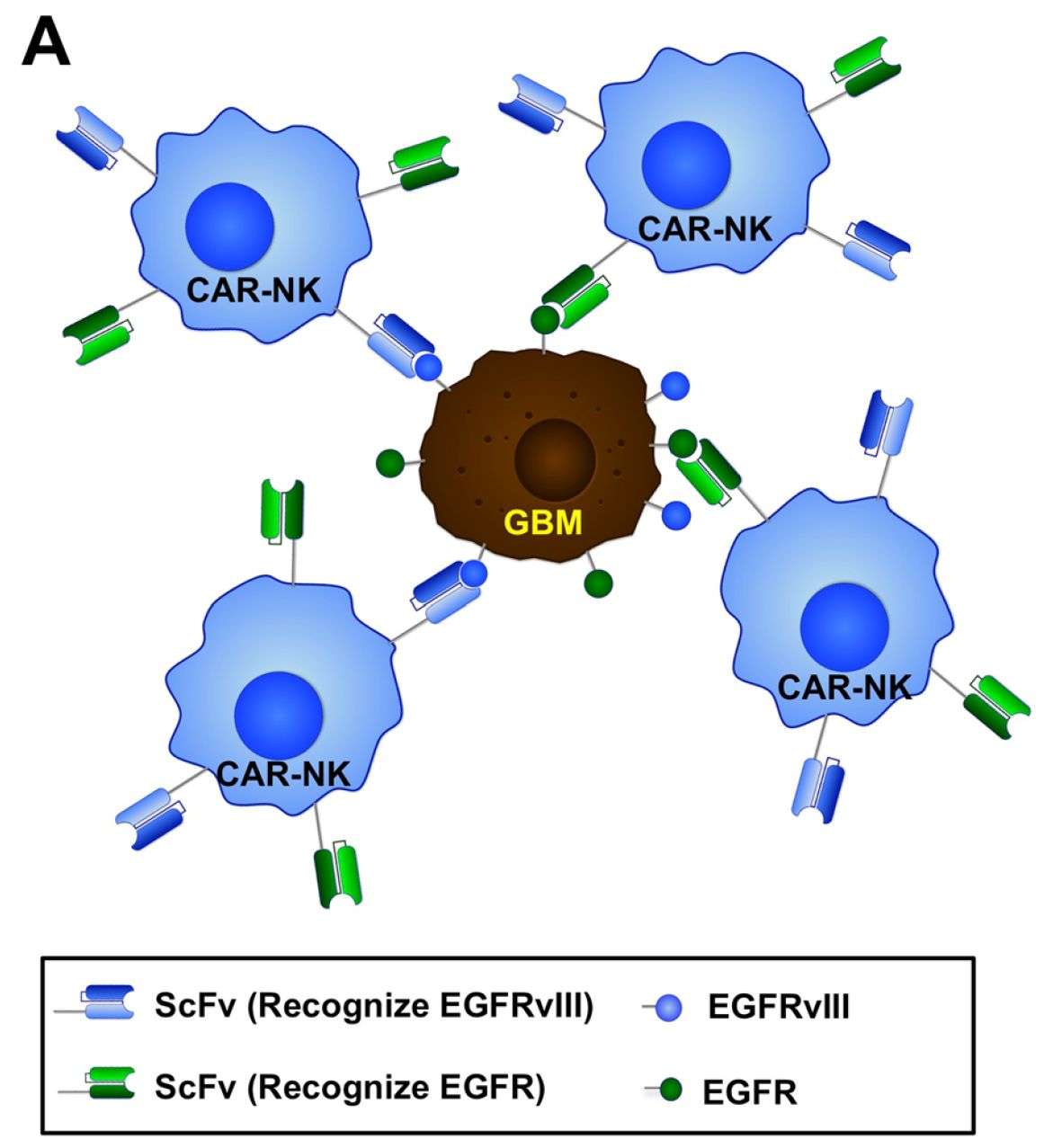 Fig 3. EGFR-targeted CAR-NK cells therapy in glioblastoma (Hamed,2022)
Fig 3. EGFR-targeted CAR-NK cells therapy in glioblastoma (Hamed,2022)
Empowered by our state-of-the-art CellRapeutics™ Chimeric Antigen Receptor (CAR) Technology, Creative Biolabs offers world-leading CAR-NK therapy development services, aiming to improve the ability of CAR cells to attack solid tumors. Our one-stop services covering Biomarker Identification & Selection, ScFv Generation, CAR-NK Vector Design and Construction, Virus Packaging & CAR Gene Delivery, CAR-NK In Vitro Assay, CAR-NK Preclinical In Vivo Assay, and CAR-NK Clinical Trial. We also provide CAR-NK Vector Products, CAR-CIK Cell Products, and a proprietary online system for customizing CAR and CAR cell products, which offers full options to meet all unique needs.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION