All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
CellRapeutics™ Next-generation CAR-TIL (Chimeric Antigen Receptor-Tumor Infiltrating Lymphocytes) development technology is an advanced platform that leverages genetic engineering to improve cancer immunotherapy. Our services typically involve multiple stages of development, including design, production, and pre-clinical trials. Creative Biolabs is proud to offer comprehensive Contract Research Organization (CRO) services specializing in CAR-TIL cell therapies.
Creative Biolabs is combining TIL and CAR technologies to achieve complementary effects in treatment. Specifically, we can enhance the tumor-clearing function by expanding TILs and then genetically modifying them to possess both tumor specificity and the antigen recognition capabilities of CAR. This combination strategy may improve the infiltration ability and persistence of T cells in the tumor microenvironment. Currently, we are actively exploring and implementing this combined approach in our labs, and we look forward to developing more effective cancer treatments in the future.
We offer services for the collection of tumor samples and the isolation and expansion of tumor-infiltrating lymphocytes (TILs). We utilize viral vectors or other methods to genetically modify TILs, enabling them to express specific chimeric antigen receptors (CARs). Additionally, we provide functional testing, activity assessment, and purity evaluation for the modified CAR-TILs. Furthermore, we can assist clients in designing and conducting preclinical studies to evaluate the efficacy and safety of TIL-derived CAR T cells in various animal models, including mice.
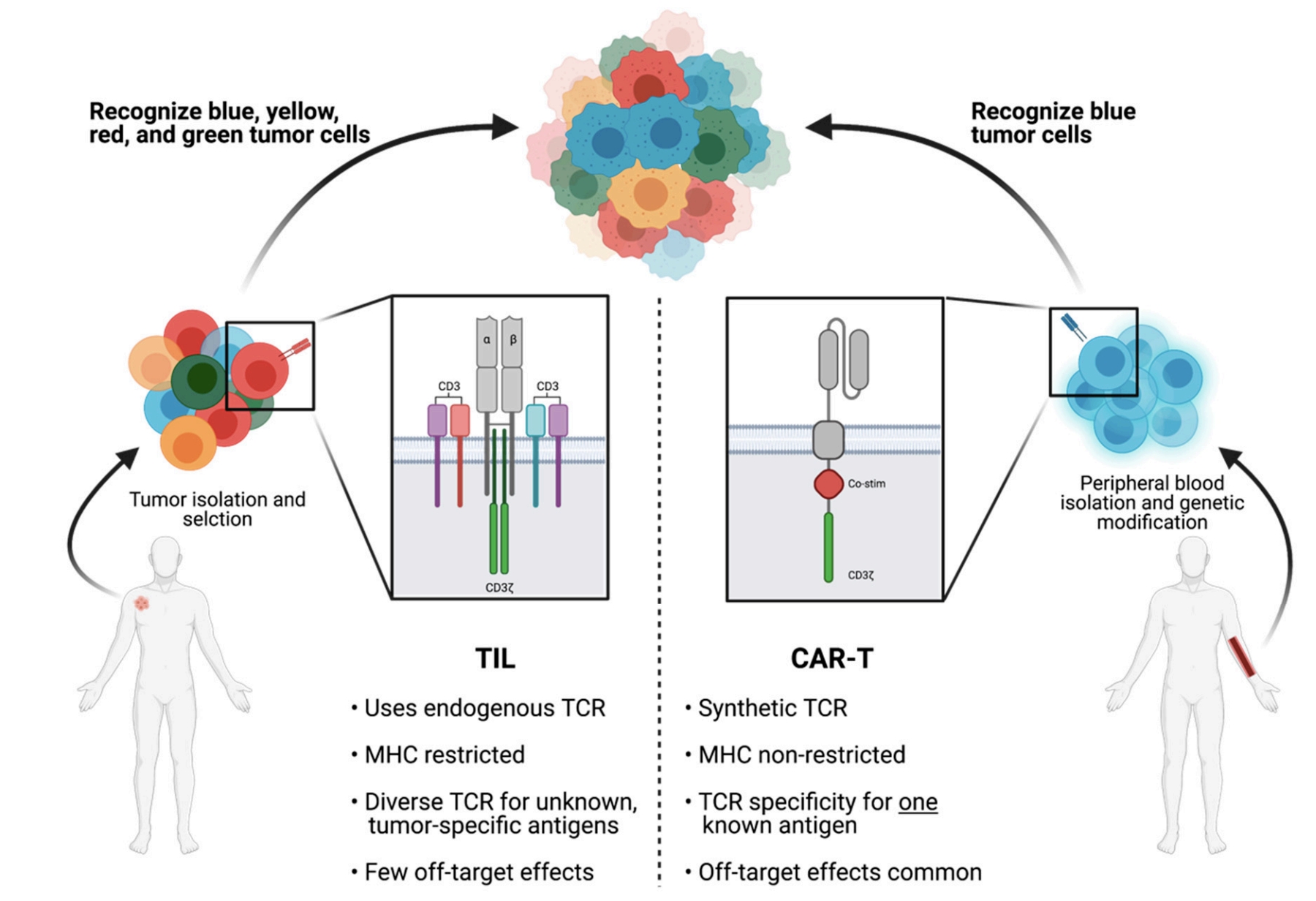 Fig.1 Comparative Characteristics of TILs and CAR-Ts in Cancer Therapy.1,3
Fig.1 Comparative Characteristics of TILs and CAR-Ts in Cancer Therapy.1,3
Recently, Creative Biolabs has developed an advanced CAR-TIL cell therapy strategy that integrates the characteristics of TILs with CAR technology, aiming to enhance the ability to recognize and attack tumors. While TILs are more effective at identifying tumor cells, their numbers are often limited, and there are challenges associated with their expansion and application in vitro. By utilizing transduction techniques to enable the expression of specific tumor-recognizing antigens, we can effectively address these challenges and create various CAR-TILs, such as dual CAR-TILs.
1. Tumor Sample Collection
Obtain tumor samples from the tumor tissue within the patient.
Collect fresh or frozen tumor tissue through surgical procedures, biopsies, or other methods.
Extraction and expansion of tumor-infiltrating lymphocytes.
2. T Cell Isolation and Expansion
Employ enzymatic techniques, like collagenase, to completely separate individual cells from the tumor tissue.
Then, isolate T cell subpopulations using flow cytometry or other cell separation methods.
Expand T cells in specific culture media to achieve a sufficient cell quantity.
3. CAR Design and Transduction
Design and synthesize CARs based on target antigens (tumor-specific antigens).
Clone the CAR gene into a suitable plasmid and utilize viral vectors (such as lentivirus or adenovirus) to transduce T cells with the CAR gene.
Enhance transduction efficiency through electroporation or chemical transfection methods.
4. Activation and Expansion of T Cells
Co-cultivate the transduced T cells with anti-CD3/CD28 antibodies to activate cell function.
Continue expanding the cells in specific culture media to generate a large quantity of CAR-T cells through multiple passages.
5. Quality Control of Cells
Perform quality assessments on the expanded CAR-T cells, including:
Detection of surface markers (to confirm CAR expression).
Evaluation of cell viability and proliferation capability.
Testing for potential contamination.
6. Preparation of Cell Products
Prepare the qualifying CAR-T cell product for clinical application, typically under GMP conditions.
Utilize cryopreservation techniques to store the cells for future infusion.
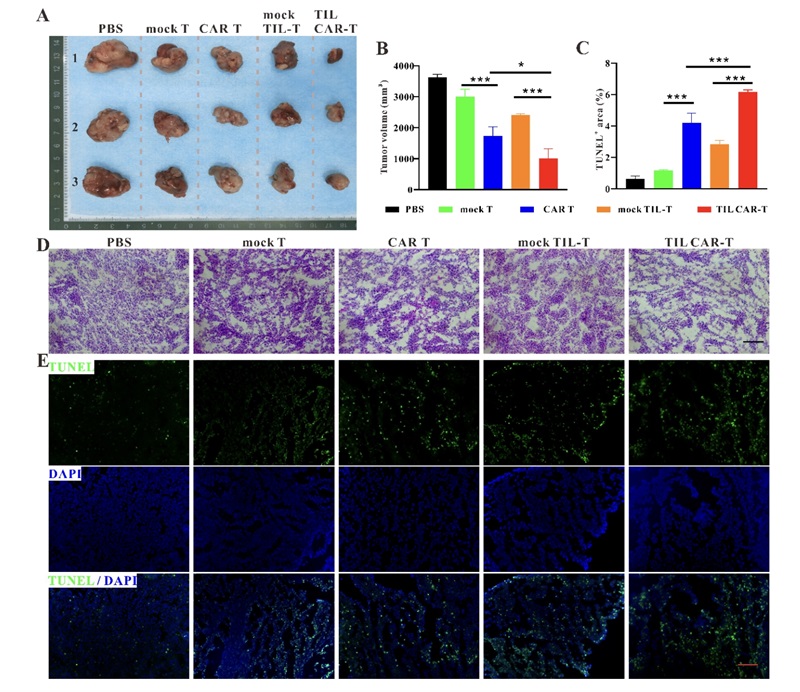 Fig.2 Tumor Growth Suppression by CAR-TIL Therapies.2,3
Fig.2 Tumor Growth Suppression by CAR-TIL Therapies.2,3
TILs are a natural part of the immune system, residing within tumors and possessing the inherent ability to recognize and attack cancer cells. By harnessing these powerful cells and engineering them with CAR technology, we can create a potent therapeutic solution that is both personalized and highly effective against specific tumor types. This innovative combination allows for:
With our comprehensive CAR-TIL development services, Creative Biolabs is committed to transforming the landscape of immunotherapy for clients around the world. If you need consultation or have any particular questions, feel free to contact us.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION