In the field of cancer treatment, tumor-infiltrating lymphocyte (TIL) therapy has emerged as a promising cutting-edge immunotherapy. To better fight against cancer, Creative Biolabs is proud to introduce its CellRapeutics™ next-generation TIL development services. Using our self-developed CAR-TIL/TCR-TIL construction platform, we can quickly design and produce TIL cells that target specific tumor antigens.
TIL therapy has been regarded as an innovative strategy for cancer immunotherapy that has garnered increasing attention in recent years. TILs typically possess a strong ability to recognize and respond to tumors, which makes them potentially very effective in treating certain resistant or advanced cancers.
Creative Biolabs offers a comprehensive range of services for CellRapeutics™ Next-Generation TIL development, which includes the establishment and characterization of tumor cell lines, isolation and culture of T cells, immune assays, and functional evaluations. Initially, we assist clients in creating suitable models to ensure the efficacy and safety of TIL therapies. Moreover, we provide precise animal studies targeting various cancer types, assessing the anti-tumor effects of TILs and their underlying mechanisms. Furthermore, we can support drug metabolism, toxicology assessments, and statistical analysis of preclinical data, establishing a solid foundation for subsequent clinical trials. By collaborating closely with us, our clients can accelerate the development process of TIL therapies, enhance success rates, and offer more
1. Sample Collection
- Acquisition of Tumor Tissue: Tumor tissue samples are obtained from patients, typically through surgical procedures or biopsies.
- Pathological Assessment: The tumor tissue is subjected to pathological evaluation to confirm the type of cancer and its characteristics.
2. Isolation and Expansion of TILs
- Isolation of TILs: Lymphocytes are isolated from the tumor tissue using mechanical or enzymatic methods, with collagenase digestion being a common technique.
- Expansion Culture: The isolated TILs are cultured in specific media, often supplemented with cytokines (like IL-2) to enhance lymphocyte proliferation.
3. Phenotypic Analysis of TILs
- Flow Cytometry Analysis: Flow cytometry is employed to analyze the surface markers of TILs, providing insights into their functional state and subpopulation composition.
- Cell Function Testing: The ability of TILs to secrete cytokines, exhibit cytotoxicity, and proliferate is assessed to evaluate their anti-tumor potential.
4. Gene Modification of TILs
- Gene Editing Techniques: Gene editing technologies can be utilized to modify TILs, enhancing their anti-tumor capabilities, for instance, by introducing specific TCRs.
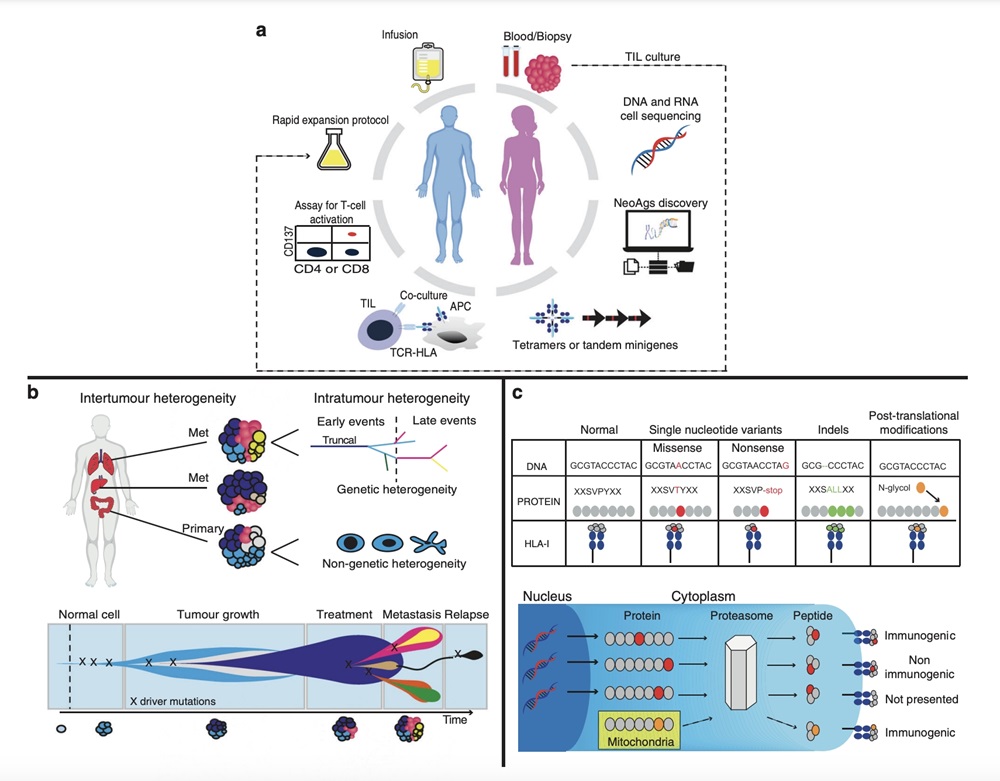 Fig.1 Tumour-infiltrating Lymphocytes for Cell Therapy Development.1,3
Fig.1 Tumour-infiltrating Lymphocytes for Cell Therapy Development.1,3
Nowadays, Creative Biolabs combines TIL with other similar cell therapies, such as CAR-T and TCR-T, to explore their synergistic effects and maximize anti-tumor efficacy. With the progress in CellRapeutics™ Next-Generation TIL therapy, we can also provide comprehensive CRO services that encompass everything from cell culture and expansion to cell phenotyping, functional assessment, and research involving animal models.
CellRapeutics™ Next-Generation CAR-TIL Development Services
In the development of CAR-TIL therapy, Creative Biolabs offers cell engineering services that encompass gene editing, plasmid construction, and transfection methods to generate T cells with anti-tumor properties. Our laboratory specializes in collecting tumor samples and expanding lymphocytes. We provide services that include the identification of highly effective tumor-infiltrating lymphocytes (TILs), performing clonal expansion, and assessing their cytotoxicity and specificity. Moreover, we conduct both in vitro and in vivo functional evaluations, employing animal (mouse) models to assess the efficacy and safety of CAR-TIL cells, in addition to carrying out pharmacokinetic and biodistribution studies aimed at refining treatment strategies.
CellRapeutics™ Next-Generation TCR-TIL Development Services
For the construction of TCR-TIL, Creative Biolabs can isolate TIL from various tumor tissue samples. Our scientists are dedicated to optimizing the conditions for the in vitro cultivation of TIL and conducting functional assessments. By evaluating the response capability of TIL against tumor cells, we can assist our clients in selecting the most promising cell populations. Additionally, CBL offers services for target antigen screening and TCR engineering to ensure specificity and effectiveness. We conduct cell culture and expansion using advanced bioreactor technology, which allows for the large-scale production of high-purity engineered T cells. Furthermore, we can provide a variety of functional assays, such as tests for cytotoxicity, cytokine secretion, and proliferation capacity.
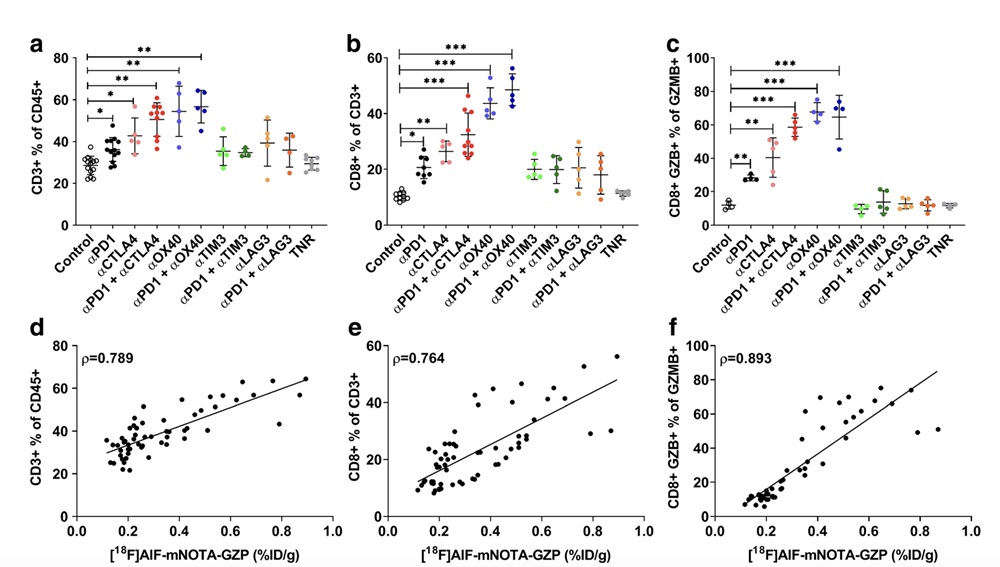 Fig.2 FACS Examination of TIL Populations in Mice with CT26 Tumors.2,3
Fig.2 FACS Examination of TIL Populations in Mice with CT26 Tumors.2,3
Synthetic Receptor based Next-Generation TIL Development Service
Synthetic receptor-based next-generation TIL therapy is a promising frontier in cancer immunotherapy. As a leader in cancer research, Creative Biolabs offers a comprehensive service to enhance the potency and specificity of TIL therapies. Our innovative approach addresses the limitations of traditional TIL therapies, such as low numbers of isolated TIL, TIL exhaustion, and the immunosuppressive tumor microenvironment.
Sensing System based Next-Generation TIL Development Service
Creative Biolabs provides an innovative sensing system based next-generation TIL development service. By harnessing advanced genetic engineering techniques, we are able to meticulously engineer TIL cells with sophisticated sensor systems that allow for the real-time monitoring of these immune cells as they navigate through the complex environment of the body. This cutting-edge approach not only facilitates the identification and selection of TIL cells exhibiting heightened anti-tumor activity but also plays a crucial role in optimizing treatment strategies tailored to individual patient needs.
Cytokine Armoring based Next-Generation TIL Development Services
Cytokines play an important role in regulating immune responses and cellular function. TILs armored with cytokines represent a significant advancement in cancer immunotherapy. To aid in cancer therapy research, Creative Biolabs delivers an innovative and robust cytokine armoring based next-generation TIL development service for clients around the world.
As a leader in the industry, Creative Biolabs offers next-generation TIL development services under the CellRapeutics™ brand. We employ advanced cell separation and amplification techniques to guarantee the collection of highly pure TIL and CAR-T/TCR-T cells. Our high-throughput screening technology allows us to rapidly identify and select TILs with the most effective anti-tumor activity. Please feel free to contact us with any questions you may have.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION