All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Creative Biolabs has developed several proprietary immunotherapy technologies, including chimeric antigen receptor (CAR)-T, TCR-T, and tumor-infiltrating lymphocyte (TIL) cell therapy techniques. We boast a robust gene sequencing analysis platform and were one of the first in the industry to fully possess self-developed immune repertoire sequencing and single-cell sequencing technologies, as well as dual CAR-TIL construction technology, which has created a unique advantage for the company in the CAR field.
TILs are a type of immune cell that has migrated to a tumor site and have shown promise in treating various solid cancers, particularly melanoma. Dual CAR-T cells can target multiple tumor antigens at the same time, which reduces the chances of tumor cells evading immune surveillance and can enhance the persistence and anti-tumor effects of T cells.
In the past few years, Creative Biolabs has aimed to enhance the efficacy of traditional CAR-T therapies by simultaneously targeting multiple tumor antigens or leveraging different TIL mechanisms of action. As a result, we have discovered a unique next-generation dual CAR-TIL development platform to improve the specific recognition and killing ability against solid tumors. Our dual CAR-TIL cell therapies integrate the functionalities of TILs (their intrinsic ability to recognize tumors) and dual CAR technology (enhanced targeting through multiple CARs).
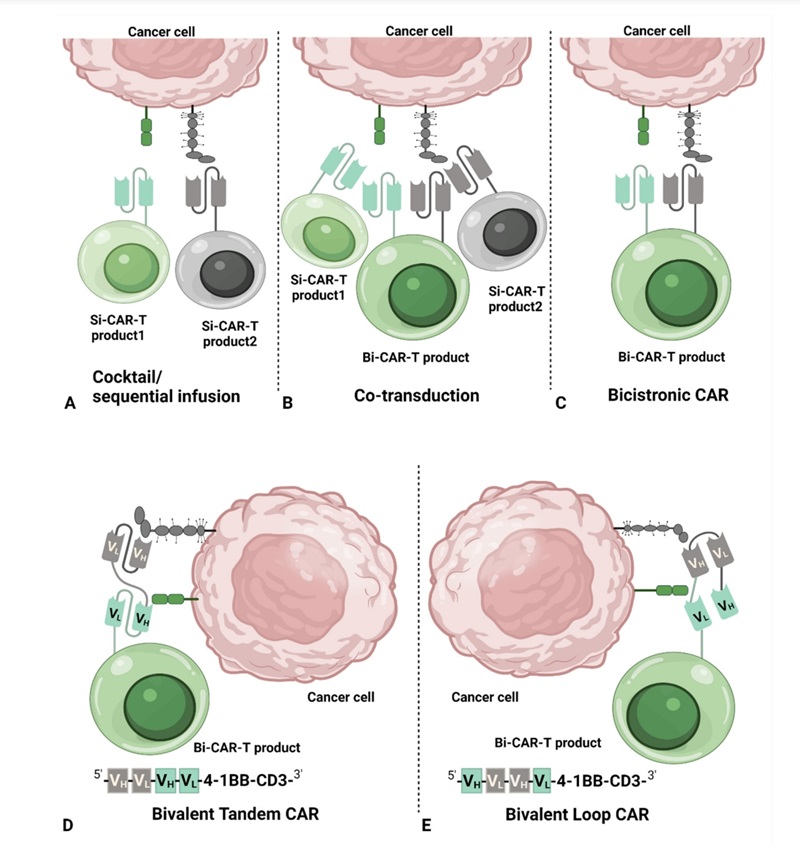 Fig.1 Dual CAR T-cell Construction Systems.1,3
Fig.1 Dual CAR T-cell Construction Systems.1,3
TIL and CAR-T technologies have played a crucial role in cancer immunotherapy. Creative Biolabs employs advanced TIL therapy techniques and comprehensive CAR construction experience to generate top-quality next-generation dual CAR-TIL cell therapy products, guaranteeing that each batch meets strict safety and effectiveness criteria.
STEP1: Acquisition of TIL:
TILs are obtained from the tumor tissue of cancer patients. This typically involves surgically removing the tumor or acquiring tumor samples through a needle biopsy.
Cell separation techniques, often employing enzyme digestion and density gradient centrifugation, are used to isolate T cells from the tumor tissue.
STEP2: Amplification of TIL:
The isolated TILs are placed into a specialized culture medium, usually supplemented with cytokines like interleukin-2 (IL-2), to promote T cell proliferation.
During the culture period, TILs can be expanded once or multiple times as needed to achieve a sufficient quantity of cells.
STEP3: Selection of Target Antigens:
Identifying Tumor Antigens: The initial step requires choosing suitable target antigens significantly overexpressed on cancer cell surfaces while being found in low levels on normal cells. This is essential for minimizing off-target effects.
Justification for Dual Targets: Opting for two antigens enhances safety, as the chances of tumor escape are decreased when two separate antigens must be expressed for the tumor cells to continue surviving.
STEP4: Construction and Transduction of Dual CAR:
For two tumor antigens, two separate CAR molecules are designed, each targeting a distinct antigen. Moreover, it must be considered whether the dual CARs will be expressed together within the same T-cell (allowing for dual signaling) or if different T-cell populations will carry various CARs.
Viral vectors (such as lentivirus or adenovirus) are utilized to introduce the constructed CAR genes into the expanded TILs, typically through transfection or transduction methods.
STEP5: Selection and Amplification of Dual CAR T Cells:
After transduction, T cells expressing the CAR are selected. The selected CAR T cells undergo further amplification to ensure there are enough cells available for clinical use.
STEP6: Efficacy Assessment and Quality Control:
The capabilities of Dual CAR-TIL cells are evaluated, focusing on their capacity to identify target tumor cells, grow in number, and produce cytokines. Ensuring the safety and effectiveness of these cell products is crucial, and they must adhere to GMP standards.
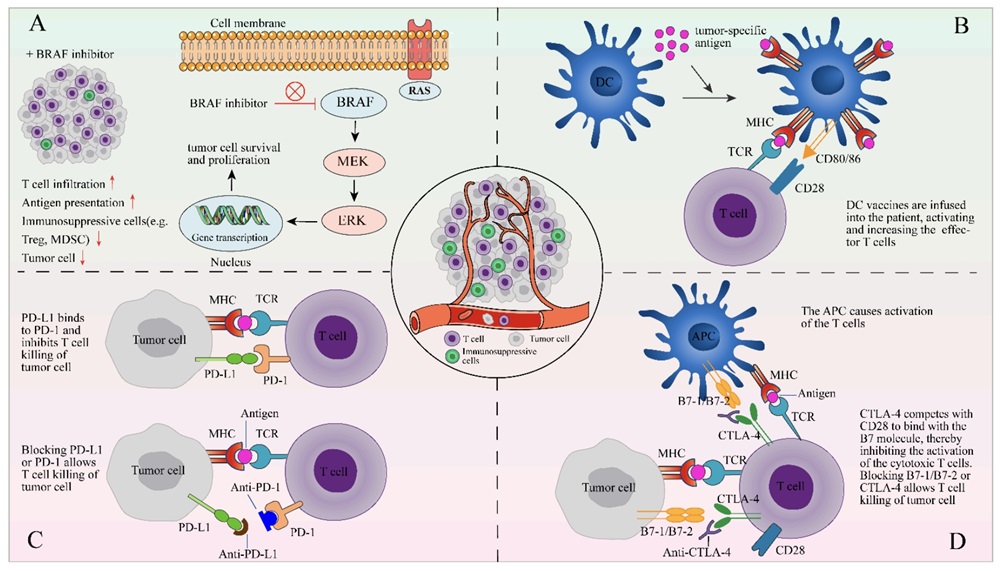 Fig.2 TIL Combination Therapies.2,3
Fig.2 TIL Combination Therapies.2,3
Key Benefits of Our Dual CAR-TIL Development Solutions:
Creative Biolabs is entering the immunotherapy market through CAR-T and TCR-T and is gradually expanding into TIL therapy by independently developing related technologies. Clients can leverage our extensive R&D experience and industry chain strategy to accelerate the commercialization process of Dual CAR-TIL therapy products, facilitating their market launch. Please feel free to contact us at your convenience.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION