All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
T-cell Receptor Therapy (TCR-T) is an innovative approach that enhances a patient's T-cells, enabling them to more effectively recognize and combat cancer cells. In contrast, tumor-infiltrating lymphocyte (TIL) therapy harnesses the immune cells that have already infiltrated tumors in the body. By combining these two advanced techniques, Creative Biolabs offers a robust CellRapeutics™ Next-Generation TCR-TIL technology that has demonstrated significant efficacy, especially in the treatment of melanoma and specific types of solid tumors.
TCR-T and TIL therapy are two advanced forms of cell-based immunotherapy that have garnered significant attention in cancer treatment in recent years. TCR-T enables the T-cells to recognize and attack tumor cells, particularly those that express particular antigens. These TILs often have a strong ability to recognize tumors since they are derived from the tumor microenvironment and can directly target a variety of tumor antigens.
Creative Biolabs combines TCR-T and TIL therapies to harness their potential synergistic effects, enhancing the immune response against tumors. Here are some of the potential technical advantages of this combined approach:
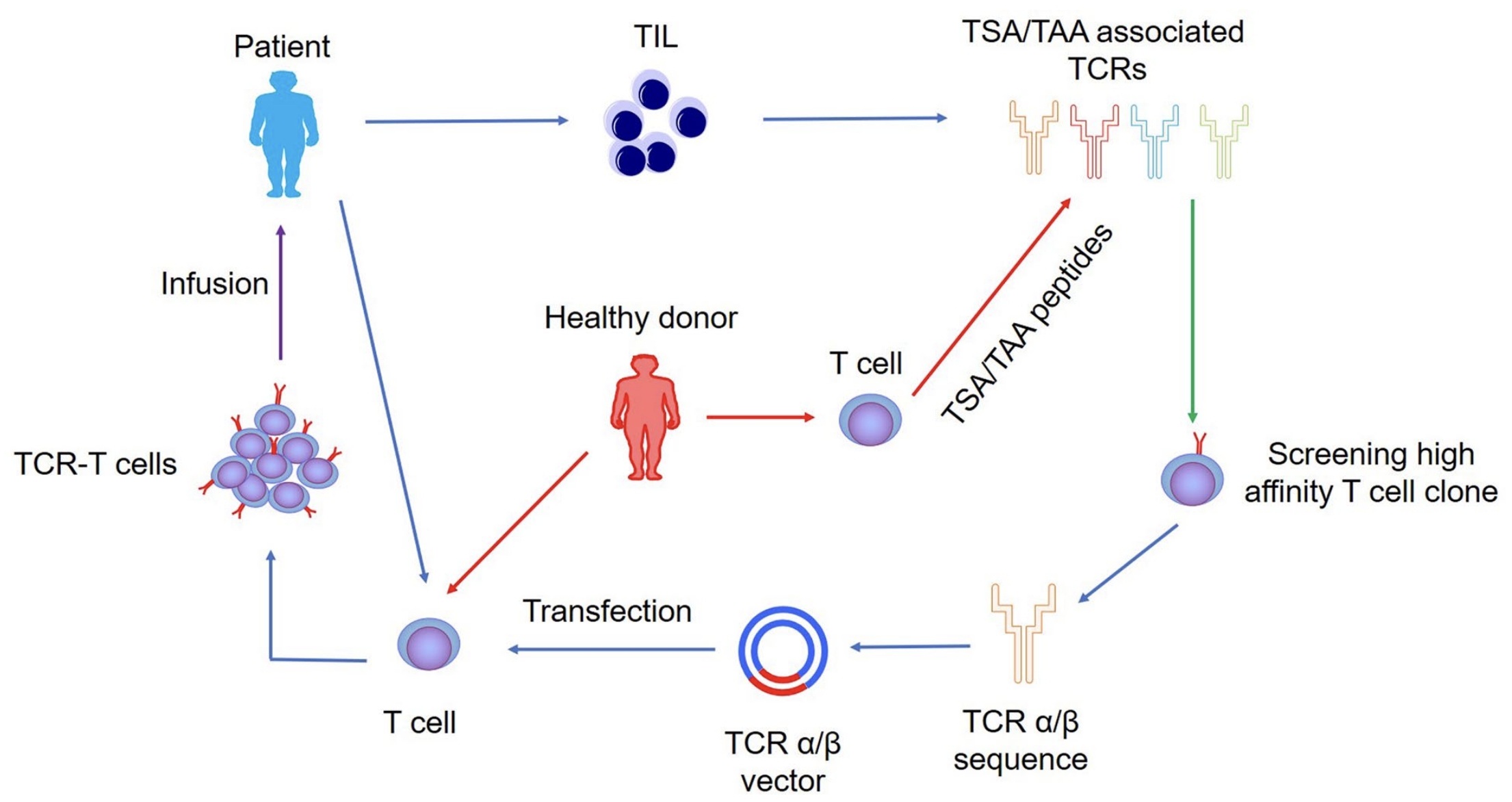 Fig.1 TCR-TIL Cell Construction.1,3
Fig.1 TCR-TIL Cell Construction.1,3
Currently, Creative Biolabs has established a TCR-TIL technology platform. Our team aims to assist clients in gaining a deeper understanding of TCR-TIL design, development, and production through a range of diverse services, as well as its potential applications in various types of tumors, such as melanoma and lung cancer. In addition, we are continuously exploring solutions to improve the survival time of TCR-TILs and to assess their toxicity, safety, and efficacy.
High-Affinity TCR Development Services
At Creative Biolabs, the development of high-affinity TCRs has emerged as a crucial element in the success of constructing TCR-engineered TILs. This process usually consists of tailored procedures aimed at enhancing the specificity and binding strength of TCRs to specific antigenic targets, like those associated with tumors or infectious diseases.
STEP1: Screening and Cloning of TCR Sequences:
- Isolate mRNA from activated T cells and use reverse transcriptase to convert it into cDNA.
- Employ PCR to amplify the TCR's alpha and beta chains, selecting high-affinity TCR genes.
STEP2: Affinity Engineering of TCRs:
- Modify the TCRs using techniques such as site-directed mutagenesis and random mutagenesis to enhance their affinity for specific antigens.
- Utilize display technologies (such as phage display and yeast display) to screen for TCRs with improved affinity.
STEP3: Transfection and Expression:
- Transfect the modified TCR genes into suitable cell lines (such as 293T or CHO cells) for expression.
- Assess TCR expression levels using techniques such as Western blotting or flow cytometry.
STEP4: Functional Validation:
- Conduct in vitro cytotoxicity assays to evaluate the cytotoxic effects of high-affinity TCR-T cells (e.g., by measuring cytokine secretion through CFSE staining or ELISA).
- Perform in vivo experiments in animal models to observe the anti-tumor efficacy of TCR-T cells.
In the development of TCR-TIL, optimizing the TCR is a crucial step. The TCR optimization services we offer typically encompass the following aspects:
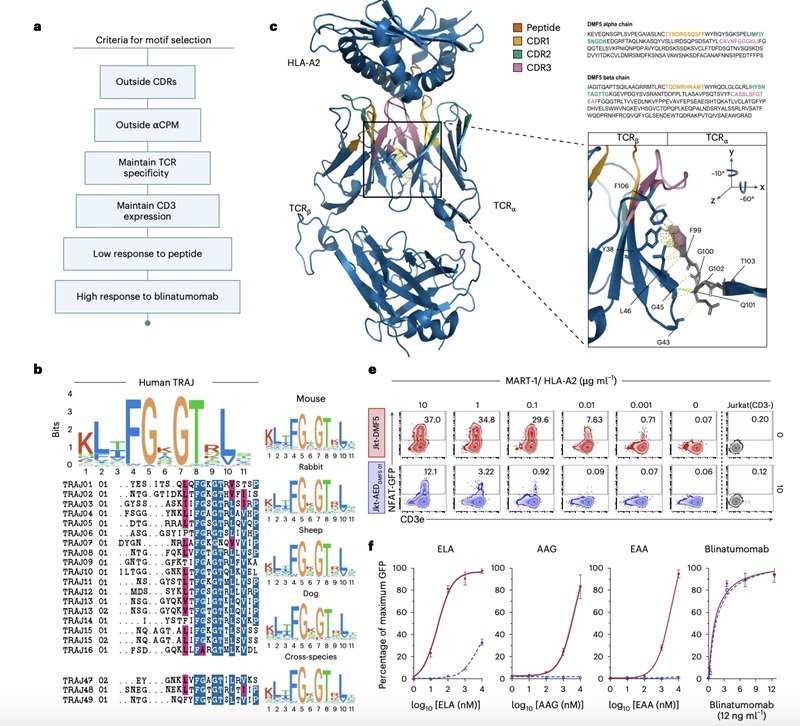 Fig.2 Analysis of TCR Sequences and the Separation of TCR-Antigen Interaction from CD3 Signaling.2,3
Fig.2 Analysis of TCR Sequences and the Separation of TCR-Antigen Interaction from CD3 Signaling.2,3
At Creative Biolabs, our TCR-TIL is a promising strategy that may offer new hope for difficult-to-treat solid tumors. This approach has several potential advantages, including:
Creative Biolabs provides TCR-TIL services, an innovative method that utilizes the immune system's strengths to fight cancer more efficiently. Our technology enhances the detection of tumors and harnesses the natural killing abilities of TILs, resulting in a stronger response against tumors. We appreciate your interest and are excited about the possibility of working together on your TCR-TIL projects. Contact us today to find out more or to begin your order!
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION