Product List Background
Background
Cholangiocarcinoma (CCA) encompasses a diverse spectrum of tumors arising throughout the biliary tract. The development of CCA is significantly linked to chronic inflammation of the biliary epithelium, a prominent risk factor often observed in conditions such as primary sclerosing cholangitis (PSC). CCA is distinct in its occurrence within the hepatic parenchyma or the large intrahepatic and extrahepatic bile ducts, each supported by distinct stem cell environments: the canals of Hering and the peribiliary glands, respectively. Similar to other gastrointestinal cancers, the pathogenesis of CCA involves the expression of activation-induced cytidine deaminase (AID) triggered by inflammatory processes affecting the bile ducts.
Signaling Pathways of CCA Development
CCA frequently develops amidst prolonged biliary inflammation and cholestasis, which drive carcinogenesis. Tumors classified under the inflammation subclass exhibit activation of immune-related signaling pathways. In contrast, the proliferation subclass is characterized by classic oncogenic pathways, including deregulated RTK signaling, RAS-RAF-ERK, PI3K-AKT-mTOR, IGFR1, MET, PLK1, AURKA, KRAS mutations, and Hippo pathway alterations. Cholangiocarcinogenesis arises from a complex interplay of extracellular ligands within the tumor microenvironment, including pro-inflammatory cytokines, growth factors, and bile acids. This interaction leads to heightened expression and aberrant activation of cell surface receptors and dysregulated intracellular signaling pathways, ultimately driving cell proliferation, survival, and genetic and epigenetic alterations. Cancer-associated fibroblasts (CAFs), recruited and activated by CCA cells and tumor-associated macrophages (TAMs), enhance CCA cell proliferation and invasion, modulate the tumor microenvironment, and induce extracellular matrix remodeling, affecting mechanotransduction and activating pathways like YAP-TAZ.
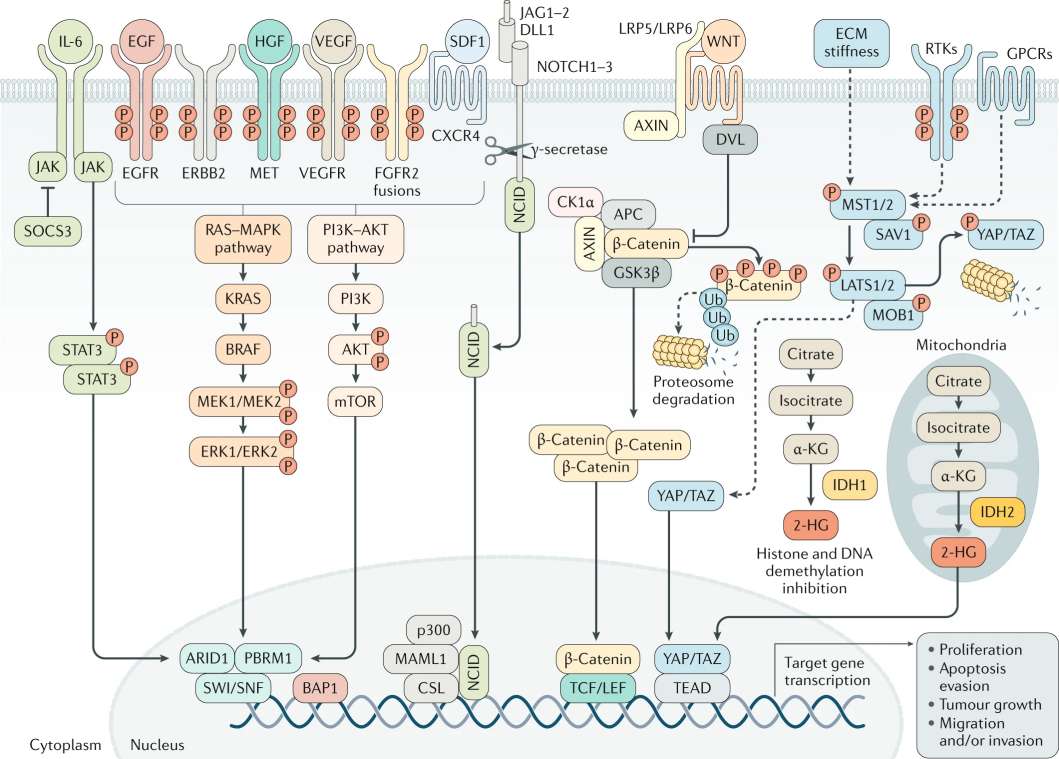 Fig.1 Signaling pathways implicated in CCA development and progression.1
Fig.1 Signaling pathways implicated in CCA development and progression.1
Utilization of CCA-Related Products
Development of Specific Aptamers for Early Diagnosis and Treatment of CCA
CCA is an aggressive biliary tract malignancy with limited options for early diagnosis and treatment, necessitating the development of effective molecular probes. Researchers have utilized cell-SELEX to develop aptamers with specific recognition for human CCA cells in a study. Researchers identify two aptamers with low nanomolar dissociation constants through sequence homology and secondary structure analysis. Further secondary structure analysis yields a truncated sequence. Using hepatocellular carcinoma cells as controls in counter selection, the aptamers exhibit high specificity for CCA cells and do not bind to other hepatocellular carcinoma cell lines. Additionally, the aptamers target membrane proteins, highlighting their potential in biomarker discovery. These aptamers may significantly contribute to the early diagnosis and clinical treatment of CCA.
Automated Microfluidic System for Enhanced CCA Detection
Conventional antibody tests like CA19-9 and CEA, commonly used in laboratory settings, lack the specificity to detect bile duct CCA, often leading to late-stage diagnoses. Researchers have developed an automated integrated microfluidic system to address this and enhance CCA diagnosis. This system automatically detects tumor cells in small bile volumes from CCA patients using three CCA-specific affinity reagents: an antibody, an aptamer, and a glycosaminoglycan, which are immobilized on magnetic beads. The system automates the entire process, including bile centrifugation and CCA capture/staining, within a short time. Single-blind tests on clinical samples have accurately identified three positive and four negative cases, indicating its potential as a promising tool for improving CCA detection.
Creative Biolabs offers a comprehensive selection of CCA cell-related products, including assay kits, aptamers, and antibodies engineered to accurately detect CCA cells. Furthermore, we provide tailored solutions, such as custom anti-CCA cell bispecific antibodies, to meet specific needs and requirements.
Reference
-
Banales, Jesus M., et al. "Cholangiocarcinoma 2020: the next horizon in mechanisms and management." Nature Reviews Gastroenterology & hepatology 17.9 (2020): 557-588.


 Datasheet
Datasheet Fig.1 Signaling pathways implicated in CCA development and progression.1
Fig.1 Signaling pathways implicated in CCA development and progression.1
