Background
Endothelial Cells
Endothelial cells line inside blood vessels and lymphatic vessels, playing pivotal roles in vascular integrity and function. They exhibit distinct characteristics such as a flat, squamous morphology and a semi-permeable barrier function that regulates the exchange of nutrients and waste between blood and tissues. Endothelial cells are involved in angiogenesis, inflammation, and immune responses, and their properties vary across different organs, contributing to organ-specific functions, such as efficient gas exchange of lung endothelial cells or blood-brain barrier maintenance of the brain endothelial cells. Aortic endothelial cells specifically maintain vascular tone, facilitate nutrient transport, and regulate blood flow dynamics critical for cardiovascular health and homeostasis. Their specialized functions are essential for the proper functioning of the circulatory system.
Inflamed Aortic Endothelial Cells
Endothelial cells transition from a quiescent to an activated state in response to stimuli like cytokines (e.g., TNF-α, IL-1β) released during inflammation, bacterial endotoxins, oxidative stress, or shear stress changes. Activation triggers endothelial cells to secrete proinflammatory cytokines (IL-6, IL-8), chemokines (CXCL1, CCL2), enzymes (COX-2), and adhesion molecules (ICAM-1, VCAM-1). These inflammatory cytokines activate multiple signaling pathways within endothelial cells, such as NF-κB, MAPK, and JAK-STAT, promoting a proinflammatory phenotype. This causes enhanced expression of adhesion molecules, facilitating leukocyte recruitment to the endothelium. Enhanced secretion of chemokines further amplifies immune cell infiltration into tissues, exacerbating inflammation.
Endothelial cells with inflammatory phenotypes contribute significantly to vascular dysfunction, promoting atherosclerosis, thrombosis, and tissue damage. They disrupt vascular integrity, increase vascular permeability, and induce oxidative stress, perpetuating a cycle of inflammation. This series of pathological changes is a driving force behind the advancement of cardiovascular disease and other inflammatory disorders.
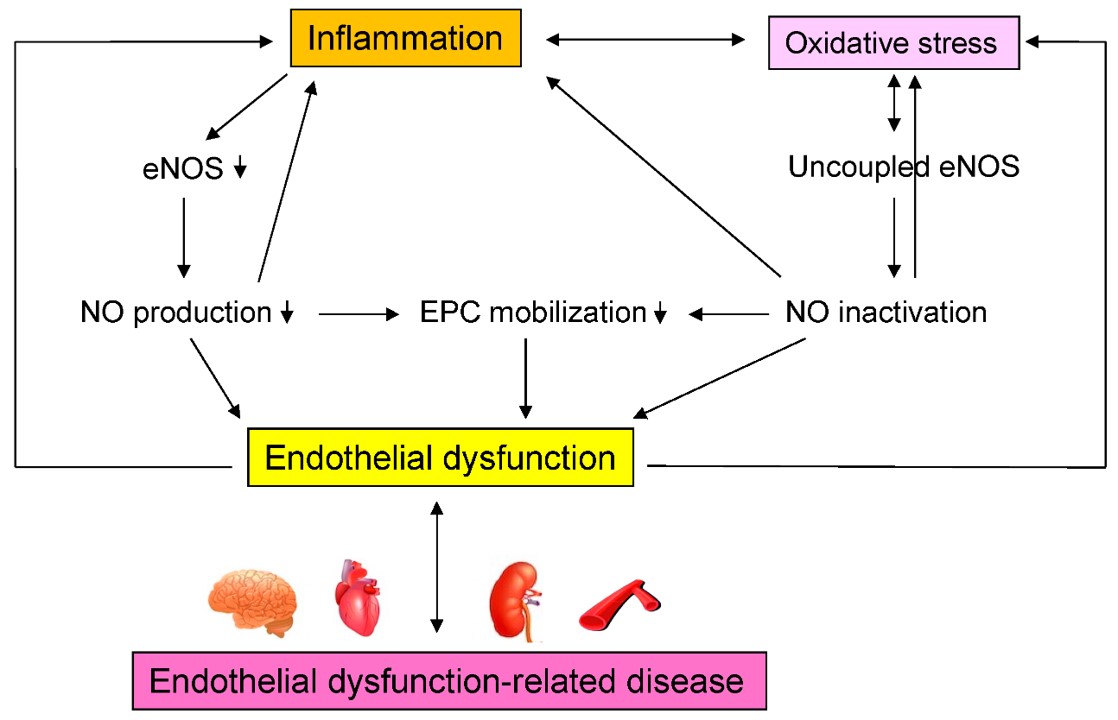 Fig.1 Mechanisms by which inflammation induces endothelial dysfunction-related disease.1
Fig.1 Mechanisms by which inflammation induces endothelial dysfunction-related disease.1
Inflamed Aortic Endothelial Cells in Diseases
Inflamed aortic endothelial cells play critical roles in various inflammatory diseases, particularly atherosclerosis. They contribute to disease progression by secreting inflammatory cytokines and chemokines, promoting leukocyte adhesion and infiltration into the arterial wall. In atherosclerosis, these cells facilitate LDL cholesterol accumulation, triggering foam cell formation and plaque development. Additionally, inflamed endothelial cells enhance vascular permeability, oxidative stress, and thrombosis risk.
Vulnerable atherosclerotic plaques are developed long before they can be detected using conventional diagnostic techniques since the early-stage plaques do not protrude into the lumen. However, these "silent" plaques can precipitate severe secondary clinical events, such as myocardial infarction and cerebral stroke, frequently contributing to morbidity and mortality. Therefore, detecting inflamed endothelial cells is crucial for diagnosing and managing cardiovascular diseases early. It allows targeted therapies to reduce inflammation, stabilize plaques, and preserve vascular function.
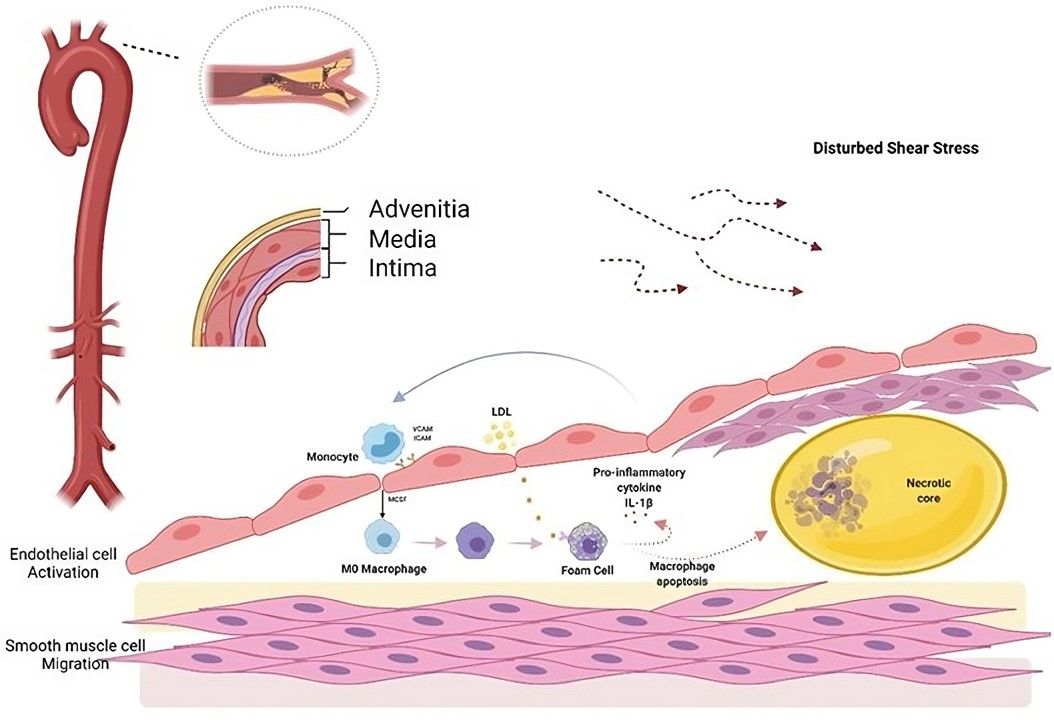 Fig.2 Atherosclerosis begins with endothelial cell activation.2
Fig.2 Atherosclerosis begins with endothelial cell activation.2
Creative Biolabs provides several aptamers targeting inflamed aortic endothelial cells, which are fully validated for complete reliability and ready support.


 Datasheet
Datasheet Fig.1 Mechanisms by which inflammation induces endothelial dysfunction-related disease.1
Fig.1 Mechanisms by which inflammation induces endothelial dysfunction-related disease.1
 Fig.2 Atherosclerosis begins with endothelial cell activation.2
Fig.2 Atherosclerosis begins with endothelial cell activation.2
