Lentiviral Vector Design for Hematopoietic Stem Cell-based Gene Therapy Development
The properties of self-renewal and multilineage differentiation capacities of hematopoietic stem cells have been widely exploited in clinical diseases treatment. Attracted by these advantages of therapy, Creative Biolabs has dedicated to gene therapy over a decade, aiming at providing comprehensive products and services with highest-grade quality. Here, we offer customized lentiviral vector design for hematopoietic stem cell-based gene therapy.
What Is Hematopoietic Stem Cell-based Gene Therapy?
Hematopoietic stem cells (HSCs) are a category of multipotent, self-renewing progenitor cells which forms differentiated blood cells and immune cells. In other words, HSCs are commonly referred to as blood or bone marrow-derived cells with potentials to renew themselves and differentiate to a variety of specialized cells. Self-renewal and multilineage differentiation characteristics pull HSCs to be a promising candidate in diseases treatment. HSC transplantation is one of the clinical application by transplanting autologous, allogeneic or syngeneic HSCs into the body to reconstruct normal cells or treat malignant and nonmalignant diseases.
Besides, HSCs-based gene therapy is a recently-developed powerful therapeutic modality to treat a growing number of human indications. In essence, HSCs-based gene therapy is a combination of gene editing and stem cell transplantation, of which the HSCs with modified-gene are transplanted into the bone marrow. The genetically engineering HSCs stably and properly proliferate and differentiate, which can reverse pathological conditions.
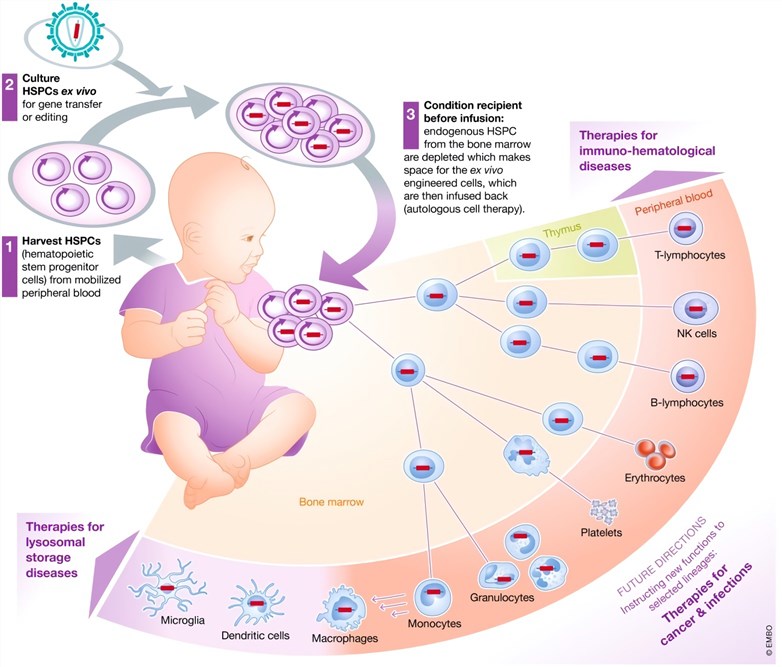 Figure 1. A schematic representation of HSCs-based gene therapy. (Naldini, 2019)
Figure 1. A schematic representation of HSCs-based gene therapy. (Naldini, 2019)
Lentiviral Vector Design for HSC-based Gene Therapy
Inserting therapeutic genes into HSCs' chromosomal DNA, transmission to progeny cells, and stable expression are the key factors of successful HSCs-based gene therapy. The prerequisite for these processes to proceed smoothly is gene transfer, which relies on a vector or delivery system. The most commonly utilized gene transfer tool is a lentiviral vector, by which the therapeutic genes can be incorporated into no matter dividing cells or non-dividing cells in a highly efficient manner. Newly developed gene transfer vector based on the human immunodeficiency virus type 1 (HIV-1), a representative lentivirus, has been widely applied for HSCs gene therapy, displaying many advantages such as efficient integration payload genes into the HSC chromatin and the decreased genotoxicity by removal pathogenetic components.
Based on these favorable superiorities, gene therapy using gene-modified HSCs by lentiviral vector has been extensively studied in a variety of human indications treatments, covering from HIV infections and immunodeficiencies, to hematological disorders, and even hereditary diseases.
Services
Aided by our well-established genetic therapy platform and experienced scientists, Creative Biolabs is proud of providing global clients with a broad spectrum of lentiviral vectors gene therapy services to help them obtain landmark development. In addition to lentiviral vectors design for HSC gene therapy, our custom lentiviral vectors also can be designed for gene silencing, cellular reprogramming, immune modulation, and gene editing. More importantly, other related lentiviral vectors services also within our scope, which include but not limited to:
- Lentiviral vector optimization
- Lentiviral vector design
- Safety determination
- Lentiviral vectors titration
- Advanced lentiviral vector development for both basic research and gene therapy
Just feel free to contact us for more detailed communications and information. We are more than happy to share our experience to help our customers in related researches.
Reference
- Naldini, L. (2019). Genetic engineering of hematopoiesis: current stage of clinical translation and future perspectives. EMBO Molecular Medicine. 11(3). pii: e9958. Distributed under Open Access license CC BY 4.0, without modification.
