Lentiviral Vector Design for Monocyte-derived Dendritic Cell
Compared with other retroviral vectors, lentiviral vectors have many advantages, including sustained expression of exogenous genes in the host, broad host range, low toxicity, and low immunogenicity. Lentiviral-mediated immunotherapy has promising applications, not only for the treatment of tumors, but also for infectious diseases, autoimmune diseases and organ transplant rejection. However, there are still many problems to be solved, such as in vivo recombination of lentivirus, virus targeting, reproductive attenuation, and non-desired mutations. To address these issues, Creative Biolabs has established a professional gene therapy team to provide customized lentiviral vector design services targeting dendritic cells (DCs).
Lentiviral Transgenic DCs in Immune Modulation
The immune system recognizes autoantigens and foreign antigens, producing tolerance to autoantigens and immune responses to foreign antigens. The specific immune response to tumors caused by the transfer of tumor-associated antigen (TAA) into DCs by viral vectors is one of the methods of tumor immunotherapy.
Highlight Functions of DCs:
- It activates mature CD4+ and CD8+ T cell lines, which migrate to tumor sites to exert anti-tumor immune effects.
- It activates naive lymphocytes in vitro, mediates strong CTL anti-tumor immune responses, and inhibits tumor formation.
- It breaks the body's immune tolerance to TAA by providing a signal that the TLR receptor interacts with the antigen while the TAA is transferred to the DC.
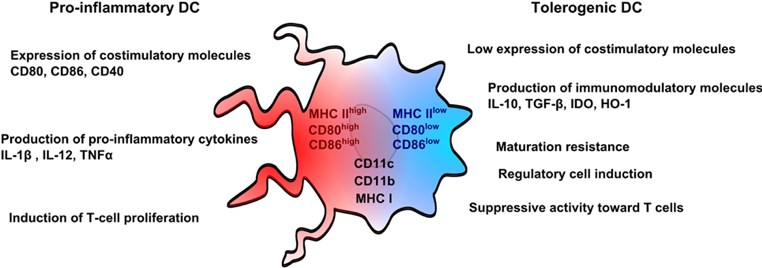 Figure 1. DC cell functions in immune modulation. (Eros, 2018)
Figure 1. DC cell functions in immune modulation. (Eros, 2018)
Given the prominent role of DC cells in immune regulation, our lentiviral transgenic DC cells service supports a wide range of applications for DC cell-based basic research, including:
- Stable transgene overexpression
- Sustained gene silencing
- Immunology
- In vivo imaging
- Production of transgenic animals
- Induced pluripotent cells
- Stem cell modification and lineage tracking
- Fixed-site gene editing
Lentiviral Vector Design Strategy for Monocyte-derived DCs
In addition to the three basic gene structures of general retrovirus gag, pol and env, the lentivirus contain four accessory genes vif, vpr, nef, vpu and two regulatory genes tat and rev. The best lentiviral vector should be a miniaturized, self-inactivation vector. Therefore, based on the different use of lentiviruses, our LV design services targeting DC cells mainly include: i) by deleting certain genes and viral proteins of the viral genome; ii) designing different expression cassettes to express replicase and envelope glycoproteins; iii) providing an envelope glycoprotein from a non-lentivirus genus. Specifically, we conduct the following designs:
- Alteration of the LTR region - this method allows efficient expression of exogenous promoters and transgenes, and allows lentiviral vectors to simultaneously express two exogenous genes.
- Introduced a sequence that is resistant to transcriptional silencing - by insertion of a forward regulatory element including local control region, chromatin insulator, and matrix attachment sites.
- Increase the transcriptional targeting of the transgene - use the promoter of the DC-specific gene as the promoter sequence of the lentiviral vector, so that the transferred gene is expressed only in a specific DC subtype.
- Add regulatory elements for transcriptional regulation - our Tet-on/ transcriptional regulation system can accurately regulate the expression level of transgenes, making LV more controllable.
Following the design of the lentiviral vector you are interested in, Creative Biolabs also conducts a series of analytical assays, such as transgenic lentivirus expression level analysis and DC activity evaluation to ensure the effect of transgenic lentivirus. Based on structural optimization, we also offer a range of downstream services such as Potency Tests, Safety and Toxicology Analysis, Delivery Systems Development, etc. to meet your needs.
Please feel free to contact us and our experienced technicians will give you the most detailed answers to your questions.
Reference
- Eros, M.; et al. (2018). Tolerogenic Dendritic Cells in Solid Organ Transplantation: Where Do We Stand? Frontiers in Immunology. 9:274. Distributed under Open Access license CC BY 4.0, without modification.
