Lymphocytic Choriomeningitis Virus (LCMV)
As a leading services provider in gene therapy, Creative Biolabs provides customized lentiviral vector pseudotyping services by lymphocytic choriomeningitis mammarenavirus based on the value of quality first, time-saving and cost-saving.
A Brief Introduction of Lymphocytic Choriomeningitis Virus
Lymphocytic choriomeningitis virus, also refer to lymphocytic choriomeningitis mammarenavirus (LCMV), is single-stranded RNA virus in Arenaviridae family, Mammarenavirus genus. LCMV is the first identified arenavirus by Charles Armstrong in 1933 as a causative agent of nonbacterial meningitis. As a worldwide distributed virus, LCMV is classified as an Old World virus whose primary host is rodent animal (mainly mouse) but also is capable to infect hamsters, dogs, pigs, primates, and even humans. For the human, LCMV is a common pathogen causing substantial neurological disorders, such as meningitis, encephalitis, and neurologic birth defects.
LCMV is a negative-sense arenavirus with the ability to infect a broad range of different cell types, including dendritic cells, tumor cells, neural cells, etc., by binding to the α-dystroglycan receptor. This characteristic of LCMV can be developed for the tropism improvement of gene therapy.
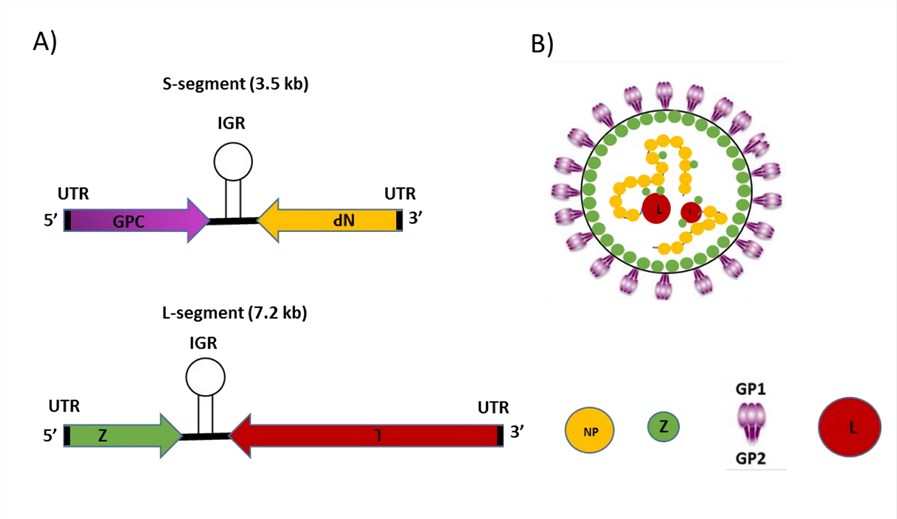 Figure 1. The genome and virion structure of Mammarenavirus LCMV. (Hussein, 2019)
Figure 1. The genome and virion structure of Mammarenavirus LCMV. (Hussein, 2019)
Pseudotyping of Lentiviral Vector by LCMV
Pseudotyping is a favorable glycoprotein optimization method to improve the transduction efficiency and stability of lentiviral vector by combining the foreign viral envelope proteins with the lentiviral vector. Pseudotyping lentiviral vectors using arenavirus, commonly glycoprotein of LCMV, have been demonstrated to infect several cell types, including hepatoma cells, neuronal cells, glioma cells, epithelial cells, etc. Additionally, LCMV pseudotyped lentiviral vectors are much safer due to non-cell toxicity of LCMV envelope glycoproteins. Furthermore, the LCMV enters cells by binding to α-dystroglycan, an extracellular matrix protein receptor that mainly expressed on dendritic cells, which makes LCMV pseudo-lentiviral vectors more easily recognized by the immune system.
Applications of LCMV-pseudotyped lentiviral vectors have been reported by many investigations. Lentiviral vectors are more tend to infect cancer cells after being pseudotyped by LCMV glycoproteins. It has been indicated that LCMV-pseudotyped lentiviral vectors could efficiently transduce solid glioma cells and specifically transduce infiltrating tumor cells (Miletic, 2004). LCMV-pseudotyped lentiviral vectors also exhibit potentially targeting neural stem/progenitor cells in vivo. Besides, alternative pseudotyping lentiviral vectors by LCMV can minimize adverse vector effects, such as hepatic injury and systemic injury.
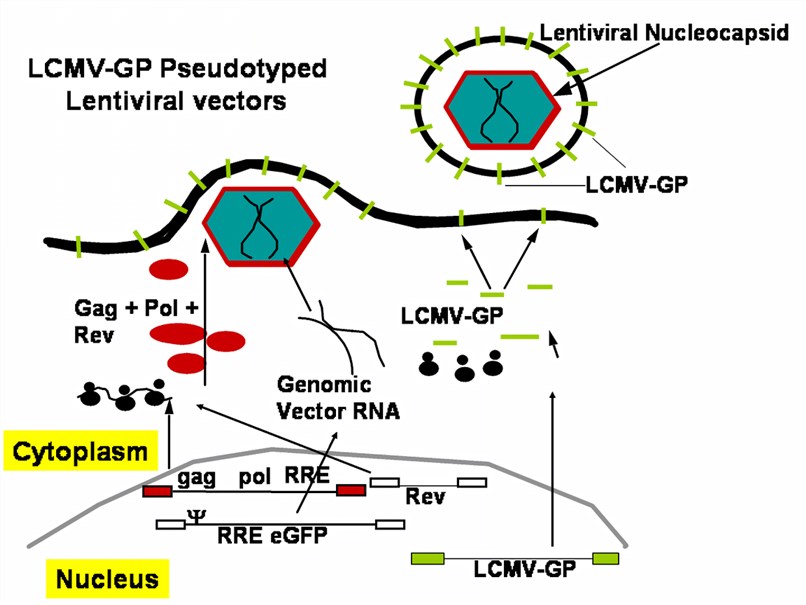 Figure 2. A packaging cell line for LCMV-GP pseudotyped lentiviral vectors. (Miletic, 2008)
Figure 2. A packaging cell line for LCMV-GP pseudotyped lentiviral vectors. (Miletic, 2008)
Features
- Different tropisms based on customers' needs
- Various types of viruses are available
- Scientists specialized in virology and gene therapy
- One-stop streamlined services from design of gene sequence and vectors, to efficient tests and data interpretation
Creative Biolabs has successfully completed many lentiviral vector-based gene therapy projects. Based on this abundant experience and advanced technology platform, we are excited to offer our tailored lentiviral vector pseudotyping services to help you get landmark development.
If you are interested in the optimization of lentiviral vectors for gene therapy, you can directly contact us for more detailed information.
References
- Hussein, A. (2019). Characterization of novel LCMV strains in Southern Iraq. University of Helsinki.
- Miletic, H. (2008). Gene therapy of malignant glioma with retroviral vectors and tumor-infiltrating progenitor cells. Universität zu Köln.
- Miletic, H.; et al. (2004). Selective transduction of malignant glioma by lentiviral vectors pseudotyped with lymphocytic choriomeningitis virus glycoproteins. Human Gene Therapy. 15(11): 1091-1100.
