MPS IIIA/Sanfilippo A
Inquiry NowWith the increasing use of adeno-associated virus vectors (AAV) in gene therapy research, Creative Biolabs' AAV vector design platform has also improved in all aspects. The excellent safety and the efficient transduction into a wide range of target tissues make AAV one of the preferred carriers for gene therapy in vivo. However, the development and screening of safe and efficient gene therapy vectors is a long process that requires adjustments to the pathological characteristics of various diseases. Our team of experienced scientists provides AAV vector design services for the treatment of mucopolysaccharidosis type IIIA (MPS IIIA) to meet your professional needs.
Introduction to MPS IIIA
MPS is a type of lysosomal storage disease (LSD) caused by the accumulation of undegraded glycosaminoglycans (GAGs) in various tissues and organs after lysosomal enzyme deficiency. Seven types of MPS are classified according to the lack of specific enzymes, accompanied by various symptoms including central nervous system (CNS) damage, hearing loss, respiratory hazard, valvular heart disease, hepatosplenomegaly, and the like. If there is no effective treatment, the patient usually dies within a few decades. MPS IIIA, also known as Sanfilippo A syndrome, is an autologous recessive neurodegenerative metabolic disease caused by sulfoglucosamine sulfohydrolase (SGSH; sulfamidase) deficiency, resulting in the accumulation of undegraded heparan sulfate (HS) in the organelles, triggering cells disfunction.
Currently, MPS IIIA gene therapy can be divided into two main approaches: one is in vivo gene therapy, which involves injecting the virus carrying the gene intravenously or locally delivering the gene to the patient's body cells; the second is in vitro gene therapy, in which genes are transferred to somatic cells from the patient and then transplanted back into the patient. Recombinant AAV (rAAV) is a common vector for gene therapy for the treatment of MPS. It can effectively infect different cell types, persist as an episome, and has a low risk of insertional mutagenesis and genotoxicity.
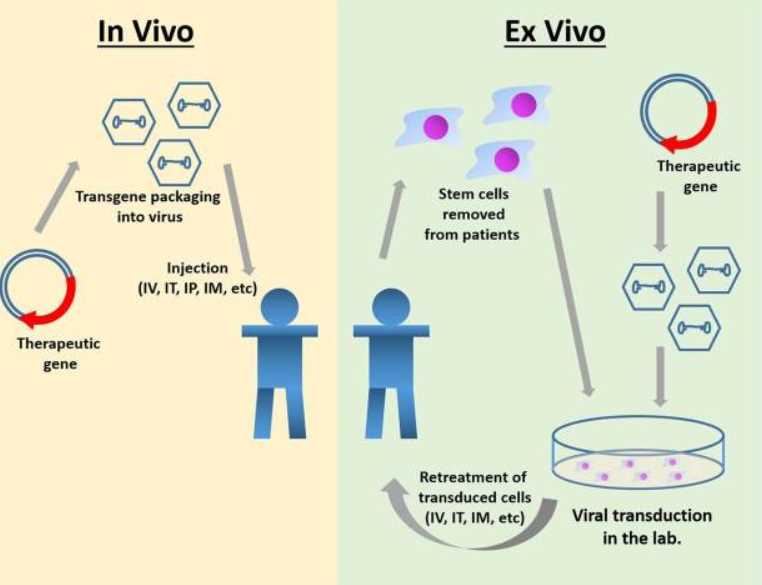 Fig.1 Gene therapy for MPS IIIA.1
Fig.1 Gene therapy for MPS IIIA.1
rAAV Gene Therapy for MPS IIIA
The molecular tool library of Creative Biolabs has a variety of AAV serotypes identified from human and non-human primates. These different serotypes of AAV can interact with different receptors specifically to mediate the delivery of therapeutic genes to target specific tissues and organs. For different diseases, a suitable route of administration is required to ensure adequate contact of the viral vectors with the target cells, intraparenchymal (IP), intra-cerebrospinal fluid (CSF) and intravenous (IV) injection are available for AAV-mediated gene therapy in clinical trials of patients with MPS. Long-term data from patients with MPS IIIA treated with intracranial first-generation AAV gene therapy (LYSSAF301) showed good tolerance to direct CNS gene therapy. We mainly build a gene therapy vector for MPS IIIA based on AAV serotype rh.10 carrying human SGSH (AAVrh.10-SGSH). It has strong targeting and safety, and it has a good record in a large number of applications.
If you have any questions about our vector design service, you can contact us by email or send us an inquiry to find a complete solution.
Reference
- Nair K, Bhat A R. Applications of Gene Therapy in Dentistry: A Review Article[J]. Journal of Health and Allied Sciences NU, 2023, 13(04): 445-452. Distributed under Open Access license CC BY 4.0, without modification.

