Muscular Dystrophy
Muscular dystrophy (MD) comprises a group of genetic diseases causing progressive muscle degeneration and atrophy. It affects various muscle types, including skeletal, cardiac, and smooth muscles, leading to symptoms such as muscle weakness, stiffness, respiratory difficulties, and heart problems. MD results from mutations in genes encoding proteins crucial for muscle structure and function, such as dystrophin, sarcoglycans, and lamin A/C. Different forms of MD, like Duchenne, Becker, and Emery-Dreifuss MD, can occur based on the type and location of the mutation. The diagnosis and treatment of MD pose challenges due to the complexity of genetic mechanisms and clinical manifestations.
Clinical Manifestations
The hallmark of muscular dystrophy (MD) is progressive muscle weakness and wasting, affecting skeletal, cardiac, and smooth muscles. Symptoms vary based on the type, severity, age of onset, and progression rate. Common signs include difficulty in walking, running, jumping, climbing stairs, or rising from a seated position; frequent falls; muscle pain, stiffness, cramps, or spasms; muscle atrophy or hypertrophy; joint contractures or scoliosis; respiratory issues like shortness of breath or coughing; cardiac problems such as irregular heartbeat or enlarged heart; cognitive impairment; and vision problems like cataracts or retinal degeneration.
Clinical Diagnosis
The diagnosis of muscular dystrophy (MD) relies on a combination of clinical features, family history, laboratory tests, and genetic analysis. The goal of the diagnosis is to identify the type and cause of MD, as well as to determine the prognosis and treatment options. Various techniques are commonly used for diagnosing MD, including enzyme tests, genetic tests, muscle biopsy, heart and lung monitoring, and electromyography (EMG). These methods measure different indicators of MD, such as enzyme levels, gene mutations, protein expression and localization, heart electrical activity and structure, lung function, oxygen levels in the blood, and muscle fiber electrical activity. These techniques have varying levels of accuracy, reliability, invasiveness, cost, and availability. When used together, they provide a comprehensive MD diagnosis.
The diagnosis of MD is crucial for determining the appropriate treatment and management of the disease. While there is no cure for MD, several therapies can enhance the quality of life and prolong the survival of patients with MD. These therapies include pharmacological therapy, physical therapy, and gene therapy. Pharmacological therapy involves using drugs that can target different aspects of MD, such as inflammation, oxidative stress, muscle wasting, or gene expression. Physical therapy includes exercises and devices designed to maintain or improve muscle strength, flexibility, mobility, and function. Gene therapy employs vectors that can deliver normal copies or modified versions of genes that are defective in MD to the target cells or tissues. These therapies have varying benefits and risks in terms of efficacy, safety, side effects, accessibility, and affordability. They can be used alone or in combination to optimize the outcomes for patients with MD.
Treatment Methods
Gene therapy presents a promising approach for treating muscular dystrophy (MD) as it has the potential to restore the expression and function of proteins that are missing or abnormal in MD. This therapy involves using vectors, such as viruses or plasmids, to deliver normal copies or modified versions of genes that are defective in MD to the target cells or tissues. Several strategies are being explored for gene therapy in MD, including gene replacement, gene repair, gene editing, and gene silencing. These approaches aim to compensate for the loss or reduction of proteins involved in muscle structure and function, correct the mutations causing MD, modify the expression or splicing of genes affected by MD, or inhibit the expression of genes that are harmful or detrimental to MD.
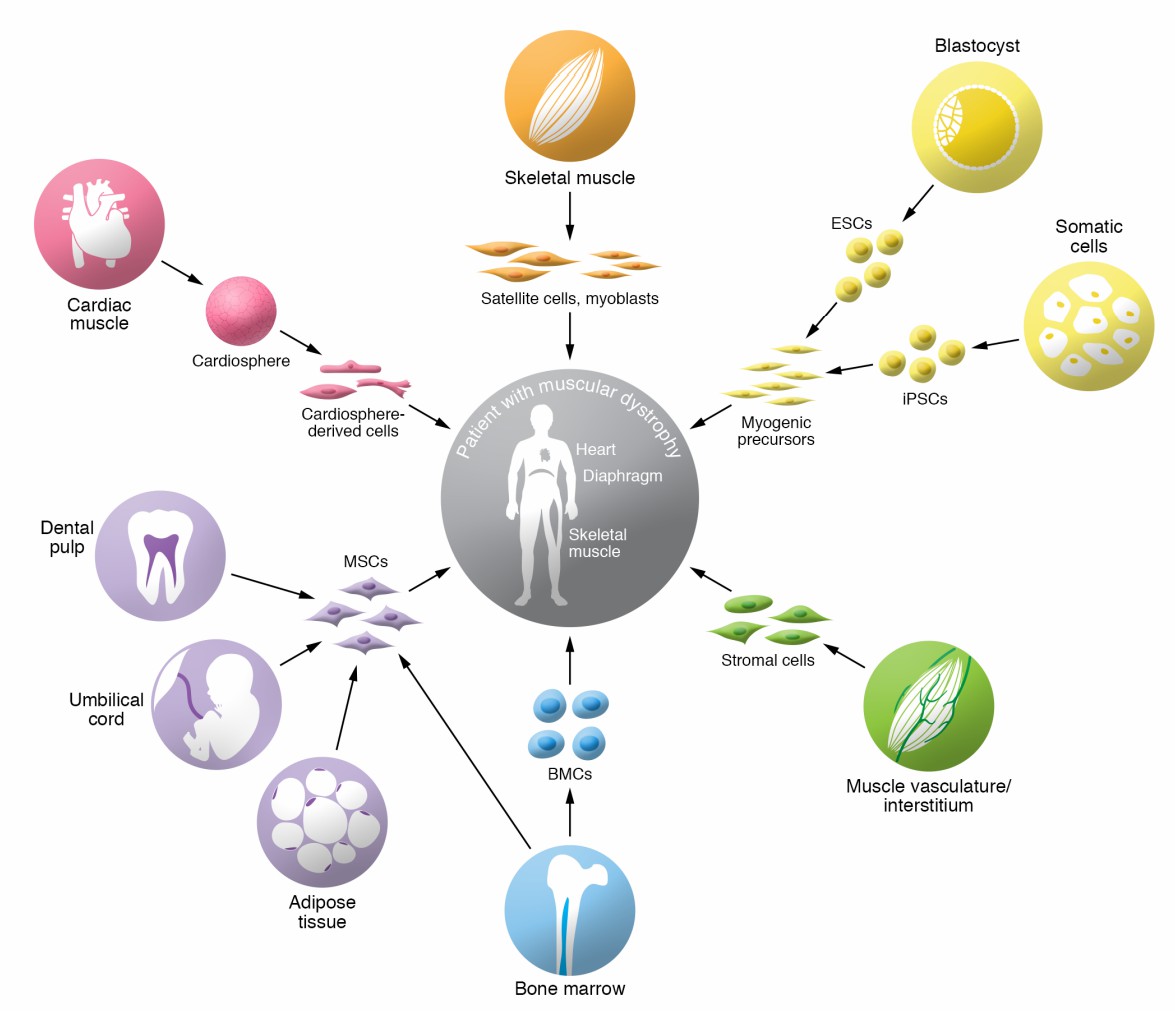 Fig.1 Overview of candidate cell types for cell therapy in muscular dystrophies. (Biressi S, 2020)
Fig.1 Overview of candidate cell types for cell therapy in muscular dystrophies. (Biressi S, 2020)
Gene therapy for the treatment of MD, however, faces numerous challenges and limitations. Some of these challenges include delivery, immune response, safety, and ethics. "Delivery" refers to the ability of vectors to reach the target cells or tissues and overcome physical and immunological barriers. "Immune response" pertains to the reaction of the body's defense system to the vectors or the transgenes and the potential consequences of inflammation, rejection, toxicity, or loss of efficacy. "Safety" encompasses the possible adverse effects of gene therapy, such as insertional mutagenesis, oncogenesis, genotoxicity, or off-target effects. "Ethics" refers to the moral principles and values guiding the conduct and evaluation of gene therapy. These challenges necessitate further research and development to overcome them and optimize gene therapy for MD.
References
- Aartsma-Rus A, et al. The importance of genetic diagnosis for Duchenne muscular dystrophy. J Med Genet. 2016 Mar;53(3):145-51.
- Abati E, et al. Adeno-Associated Virus (AAV)-Mediated Gene Therapy for Duchenne Muscular Dystrophy: The Issue of Transgene Persistence. Front Neurol. 2022 Jan 5;12:814174.
- Arechavala-Gomeza V, et al. Revertant fibres and dystrophin traces in Duchenne muscular dystrophy: implication for clinical trials. Neuromuscul Disord. 2010 Apr;20(4):295-301.
- Biressi S, et al. Stem cell therapy for muscular dystrophies. J Clin Invest. 2020 Nov 2;130(11):5652-5664.
- Bello L, Pegoraro E. Genetic diagnosis in muscular dystrophy. Curr Opin Neurol. 2012 Jun;25(3):283-9.
- Bönnemann CG, et al. Diagnostic approach to the congenital muscular dystrophies. Neuromuscul Disord. 2014 Apr;24(4):289-311.
- Bushby K, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010 Jan;9(1):77-93.
- Bushby K, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 2010 Feb;9(2):177-89.
- Flanigan KM. Duchenne and Becker muscular dystrophies. Neurol Clin. 2014 May;32(3):671-88.
- Gao QL, Zhang YH. Gene therapy for Duchenne muscular dystrophy: progress and challenges. J Zhejiang Univ Sci B. 2013 Jan;14(1):1-13.
