Quantitative and Digital Droplet-Based AAV Genome Titration
Creative Biolabs's viral vector technology platform can help customers determine the concentration of vector genomes packaged in capsid proteins in purified adeno-associated virus (AAV) preparations. Our precise and stable testing service is the most critical step before your recombinant AAV vector is put into clinical trials. It can help you determine the required dose and greatly improve your work efficiency.
Introduction of rAAV Titration
The ability of recombinant rAAV to deliver genes has shown promise in the field of gene therapy. However, before it is put into use, it is generally necessary to determine the carrier dose used in the experiment, which makes the quantitative work of carrier preparation become particularly important. The methods currently used to quantify recombinant AAV mainly include dot blot analysis, capsid ELISA or qPCR for titrating assembled particles. However, measuring only the genomic titer of vector preparations is inaccurate since the infectious titer can greatly vary that limits the use of genomic particle calculations. Other methods such as serial dilution repeated assays involving repeated infection with AAV or the use of stably transfected helper cell lines and dot blot or PCR analysis, are cumbersome and time-consuming.
In order to solve the above problems, we developed an rAAV titer detection scheme based on the life cycle of viral infection. After rAAV binding to its receptor, endocytosis and endosomal processing of AAV particles result in nuclear translocation of virions. In the nucleus, viral ssDNA is converted to transcriptionally active dsDNA by de novo synthesis of the second strand. Non-infectious viral particles either do not enter the cell or are not further processed after endocytosis. Viral DNA is stored in its single-stranded form and can be efficiently removed by digestion with ssDNA-specific S1 nuclease. The remaining viral dsDNA can be considered as DNA of transcriptionally active viral particles that can be quantified by PCR.
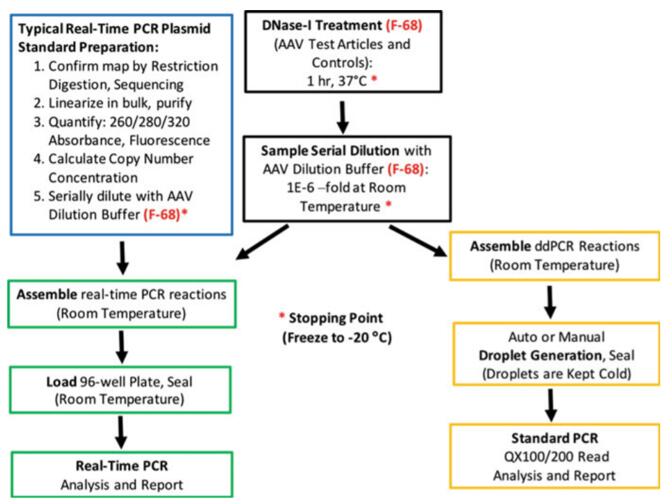 Figure 1. Protocol of Real-Time qPCR for rAAV Titration. (Sanmiguel, 2019)
Figure 1. Protocol of Real-Time qPCR for rAAV Titration. (Sanmiguel, 2019)
Services
Because the accurate assessment of rAAV titers is critical to ensuring accurate and safe carrier doses in preclinical and clinical settings, Creative Biolabs has built our vector quantitation service platform with advanced technology.
qPCR is a preferred method for the titer of the AAV genome because of its simple operation, stable experimental conditions and accurate results. However, since the primers are rejected and interfered by the annealing of the genome itself, there are many errors in determining the AAV vector genome titer by qPCR. To this end, we have developed ddPCR technology that can directly quantify DNA copies. ddPCR is a new PCR technology that directly quantifies DNA copies with unparalleled accuracy, without the need for standard curves or high amplification efficiencies; all of which help us to accurately quantify single-stranded and self-complementing AAV genomes. Absolute quantification of the single-stranded AAV vector genome by ddPCR, up to a 4-fold increase in titer compared to standard qPCR titration, with readings comparable to optimized qPCR assays.
If you have any questions about our vector titration service, you can contact us by email or send us an inquiry to find a complete solution.
Reference
- Sanmiguel, J.; et al. (2019). Quantitative and Digital Droplet-Based AAV Genome Titration. Adeno-Associated Virus Vectors. Humana Press, New York, NY. 51-83.
