Creative Biolabs is a leader in the field of biotherapeutic product development and has focused on the immunogenicity analysis for years. We have experienced experts and advanced platforms that are able to provide excellent services in this field. Our platforms have built a team of experienced scientists with facilities and processes designed specifically to provide the best strategy and protocols customized to immunogenicity evaluation.
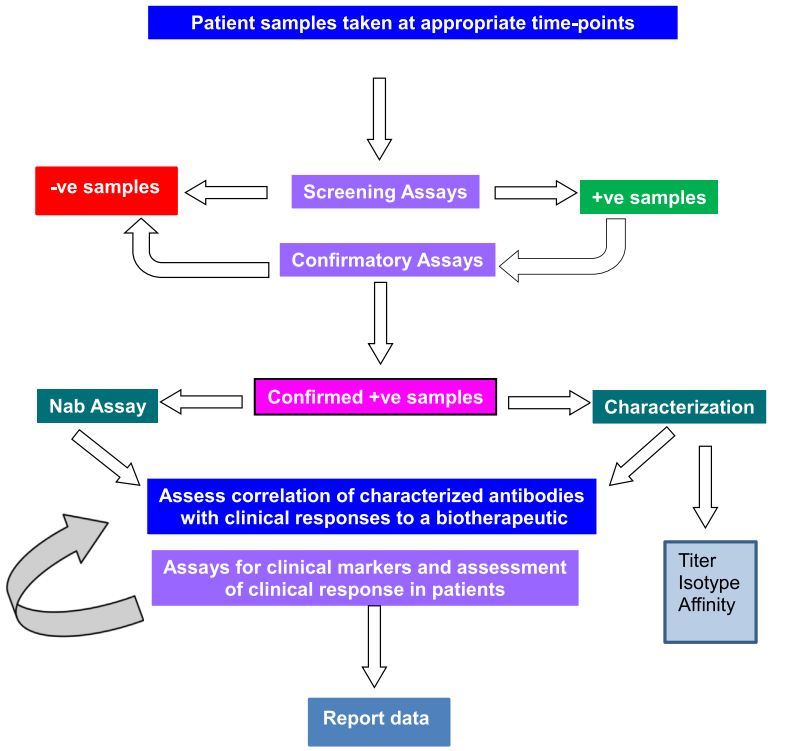
Biologic therapies have shown promising results in treating a wide collection of diseases in mammals. However, pilot studies have demonstrated that these drugs or therapeutic proteins can cause many side effects in patients or animal models. Immunogenicity has been considered as the main cause of producing anti-drug antibodies (ADAs) and thereby leading to the loss of immune responses against various diseases. Therefore, many recent studies have been focused on analyzing the immunogenicity of biotherapeutic products (BTPs). Meanwhile, since immunogenicity has been reported with many kinds of antibodies, such as monoclonal antibodies, recombinant antibodies, a full range of assays have been developed for evaluating the immunogenicity in pre-clinical trials. Currently, the common method for testing BTP immunogenicity is based on revealing the structural characteristics and functional properties. Among them, the detection and characterization of antibodies play a significant role in analyzing the safety and efficacy of a BTP. In a typical strategy, a screening assay is widely used for detecting the ability of antibody binding and neutralizing capacity. Such immunogenicity data are usually detected in the context of immune-related diseases.
 Fig.1 SIAT® Immunogenicity Analysis of Immune-related Diseases.
Fig.1 SIAT® Immunogenicity Analysis of Immune-related Diseases.
A number of biotherapeutics have been identified and broadly used in the treatment of various types of immune-related diseases, such as tumors. However, it became clear that the majority of products can trigger unwanted immune responses and then lead to a reduction or loss of efficacy in clinical trials. As a result, Creative Biolabs offers a series of SIAT® immunogenicity analysis services for evaluating the BTP in immune-related disease therapy. We have developed many animal models to provide a comparative immunogenicity assessment in pre-clinical phases. In vitro strategies for evaluating the immunogenicity have been accomplished by using in silico screening and antibody screening assays. The obtaining results suggest that these methods are rapid, reproducible and accurate in drug development. Additionally, we have also generated our SIAT® immunogenicity analysis platform basing on various disease types, including but not limited to SIAT® Immunogenicity Analysis of Tumors, SIAT® Immunogenicity Analysis of Autoimmune Disease, SIAT® Immunogenicity Analysis of Infectious Disease, as well as SIAT® Immunogenicity Analysis of Vaccines. In recent studies, the immunogenicity of a whole-gene mutation DNA vaccine targeting the Aleutian mink disease virus (ADV) has been assessed by immunofluorescent assay and enzyme-linked immunosorbent assay (ELISA).
Creative Biolabs is dedicated to delivering safe and effective biotherapeutic products to expand cutting edge research across a range of applications. With our proven competencies and regulatory expertise, we are therefore confident in offering the best immunogenicity analysis services targeting different kinds of diseases. We can provide many flexible options, from which you can always find a better match for your particular project. If you are interested in our services, please feel free to contact us for closer communication to learn how we can be involved in your project. Separate services or integrated end-to-end solutions are all welcomed.
Learn more about immunogenicity analysis services for immune-related diseases:
Immunogenicity analysis refers to the study of how the immune system reacts to therapeutic agents, particularly biologics. In immune-related diseases, this analysis helps determine whether a treatment triggers an unwanted immune response, such as the production of anti-drug antibodies (ADAs), which can reduce treatment efficacy or lead to adverse effects.
In immune-related diseases, treatments often involve biologics that can be recognized as foreign by the patient's immune system. Immunogenicity analysis is crucial because it identifies whether the body is likely to generate an immune response against these treatments, potentially neutralizing their effects or causing harmful side effects.
Immunogenicity in patients with immune-related diseases is typically assessed through serum assays that detect the presence of ADAs. These assays must be highly sensitive and specific to distinguish between ADAs and the disease’s autoantibodies, ensuring that the results accurately reflect the immune response to the drug, not the underlying condition.
Neutralizing antibodies (NAbs) are a type of ADA that can directly interfere with the biological activity of a therapeutic drug. In immunogenicity analysis, detecting NAbs is crucial because their presence can significantly undermine the effectiveness of treatments for immune-related diseases by blocking their therapeutic action.
Strategies to minimize immunogenicity include engineering the therapeutic protein to reduce epitopes that trigger immune responses, optimizing the drug formulation to avoid aggregation, and improving the purity and stability of the drug. Tolerance induction protocols and concomitant immunosuppression may also be used depending on the severity of the immune response and the patient's condition.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.