Creative Biolabs is a leading service provider that focuses on inducible pluripotent stem cells (iPSCs)-derived cell therapy services. Currently, various platforms have been established for analyzing the function of iPSC-derived macrophage in developing novel stem cell therapy. By combining cutting edge technologies with proprietary innovations, we also provide broadly applicable iPSC-derived macrophage models for a wide range of disease types.
iPSCs, a member of stem cell family, play an important role in generating a number of specific immune cells for different types of disease treatments, especially for various cancers. In the past few years, a wide variety of techniques have been developed to produce iPSCs, including different vectors. Previous studies have demonstrated that specific reprogramming factor combination has been commonly used for vector-based reprogramming for iPSC generation, also known as iPSC reprogramming factors delivery by integration-free vectors.. Meanwhile, pilot studies have indicated that iPSC can be derived from immune cells, such as macrophages, to develop new stem cell therapy for treating various genetic diseases. Moreover, recent researchers have revealed that iPSC-derived macrophages are critical for establishing macrophage polarization models and for analyzing the function of macrophages in human genetic disorders.
At Creative Biolabs, we deeply understand the requirement of our clients in iPSC-derived macrophage services. We are always dedicated to developing and improving our skills to make custom services for your target of interest. Up to now, we have generated a full range of iPSC-derived macrophage assays to offer a comprehensive iPSC-based solution for disease immunotherapy, such as differentiation services of iPSCs to macrophages, functional analysis of iPSC-derived macrophages, transcriptome analysis of iPSC-derived macrophages, iPSC-derived macrophage polarization assays, and disease modeling of iPSC-derived macrophages. Based on our advanced technologies, iPSC-derived macrophages can be regarded as unlimited genotype-specific cells to permit deeper insights into the relationship of the function of macrophages and plenty of human diseases.
Macrophages play a crucial role in the immune response and are key players in inflammation and tissue repair processes. By using iPSC technology, we are able to provide a consistent and scalable source of macrophages for a variety of research applications.
Our custom service is designed to provide researchers with a reliable and convenient source of high-quality macrophages for a variety of research applications, including drug discovery, immunology, and regenerative medicine.
With the help of abundant experience and professional scientists, Creative Biolabs is committed to guiding global customers to a series of custom iPSC-derived macrophage services. Our laboratory services have many advanced chemical and biological technologies to support customer's needs in the fast-growing iPSC-derived cell therapy research. If you are interested in our services, please feel free to contact us or send us an inquiry.
Below are the findings presented in the article related to ipsc-derived macrophage.
Simon Gutbier et al. described an improved method for the use of iPSC for the high-yield, large-scale production of cells resembling tissue-resident macrophages. They thoroughly characterized the iPSC-derived macrophage-like cells to confirm their cell identity and thus their suitability for drug screening purposes.
The results showed that these iPSC-derived macrophages shared strong cellular identity with primary macrophages and recapitulated key functional characteristics, including cytokine release, phagocytosis, and chemotaxis. In addition, they demonstrated that genetic modifications can be easily introduced at the macrophage-like progenitor cell stage to probe drug target-related pathways.
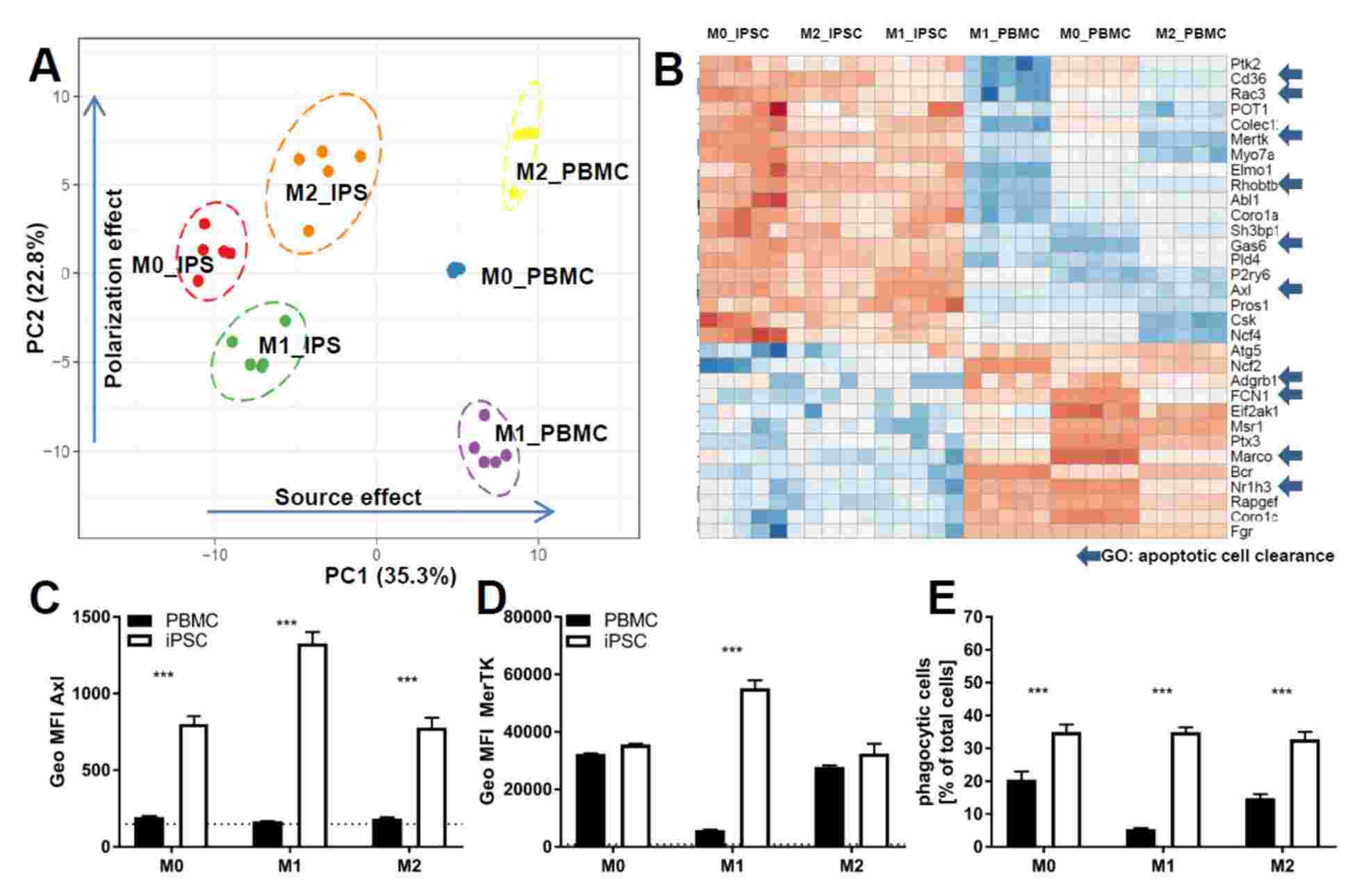 Fig. 1 Comparison of functionality of iPSC-derived and CD14+ cells derived from PBMCS.1
Fig. 1 Comparison of functionality of iPSC-derived and CD14+ cells derived from PBMCS.1
Reference
For Research Use Only. Not For Clinical Use.