Overview Service Features FAQs Scientific Resources Related Services
In the stem cells therapy, the induced pluripotent stem cells (iPSCs) which are directly derived by reprogramming patients' cells to an embryonic-like state. Since iPSCs could be coaxed into the desired cell types that would already be genetically matched with the patient, the immune rejection and the ethical issues can be potentially circumvented.
Overview of iPSC-derived Cell Therapy
There are three areas of research and the influence they had on the generation of iPSCs. Firstly, the method of nuclear trans provided the possibility of differentiated cells retain the same genetic information as early embryonic cells; Then, the development of techniques allowed researchers to derive, culture, and study pluripotent cell lines; Lastly, the observation that transcription factors are key determinants of cell fate whose enforced expression can switch one mature cell type into another let iPSCs technology become true. By the introduction of a defined and limited set of transcription factors which including octamer binding transcription factor 3/4 (OCT3/4), SRY-related high mobility group (HMG) box protein 2 (SOX2), MYC and Kruppel like factor 4 (KLF4) and by culturing these cells under ES cell conditions, the iPSCs can be generated from fibroblasts. The method is striking in that it can convert somatic cells directly into pluripotent cells by straightforward reprogramming procedure.
With the synthesis of scientific principles and technologies advancing, the induced pluripotent stem cells (iPSCs) have been developed over the last six decades. iPSCs are human somatic cells which have been reprogrammed to a pluripotent state, these cells can be considered as a potential patient-specific cell therapy or a specific model for disease research. With a sophisticated design, in Creative Biolabs, the iPSCs can be used as the pluripotent starting material for differentiated cells or tissues in regenerative medicine and they can be used as a powerful tool for immune cells treatment.
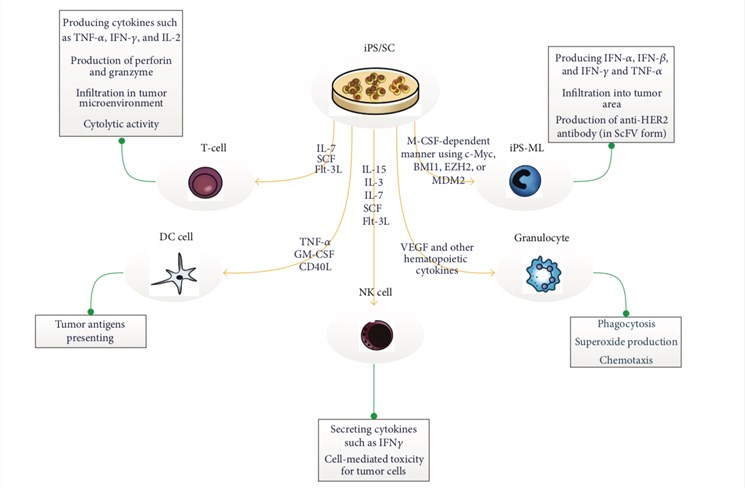 Fig.1 iPSC-derived cell therapy.1
Fig.1 iPSC-derived cell therapy.1
iPSC-derived Cell Therapy Development Services
With efforts made by our brilliant scientists, at Creative Biolabs, we provided iPSCs services to clients all over the world.
Currently, various platforms have been established to generate and analyze iPSC-derived immune cells to promote the development of novel stem cell therapy. We can provide the following custom services based on different immune cells:
Please contact us without hesitation to get more information.
Our iPSC-derived cell therapy development services include the following:
-
iPSC Generation: We specialize in generating induced pluripotent stem cells (iPSCs) from various cell sources, including fibroblasts, blood cells, and other somatic cells.
-
Differentiation Protocols: We have developed robust differentiation protocols to generate specific cell types from iPSCs.
-
Characterization and Quality Control: We perform thorough characterization and quality control studies on all iPSC-derived cell populations to ensure their identity, purity, and functionality.
-
Disease Modeling: Our iPSC-derived cell therapy development services also include disease modeling studies, where we generate disease-specific iPSC lines and differentiated cell types to study the underlying mechanisms of various diseases.
-
Preclinical Testing: We offer preclinical testing services for iPSC-derived cell therapies, including in vitro and in vivo studies to assess the safety, efficacy, and mechanisms of action of the treatments.
We are committed to excellence in research and development, and we work closely with our clients to customize our services to meet their specific needs and goals.
Features of Our Services
-
Customized Solutions
-
High-Quality iPSC Lines
-
Advanced Characterization and Validation
-
Scalable Manufacturing
-
Collaborative Research and Development
FAQs
-
Q: What kind of cell types can you differentiate iPSCs into?
A: We have expertise in differentiating iPSCs into a wide range of cell types, including neurons, cardiomyocytes, hepatocytes, pancreatic cells, and various immune cells like NK cells and T cells. We can also develop specific subtypes and organoid systems based on your research requirements. Our team stays current with the latest protocols and technologies to provide cutting-edge differentiation solutions.
-
Q: How long does it typically take to develop iPSC-derived cells for my research project?
A: The timeline depends on the complexity of your project, including factors like reprogramming, differentiation, and quality control. Generally, reprogramming cells to iPSCs can take 8-12 weeks, followed by an additional 4-8 weeks for differentiation and QC. For more complex projects, the timeline may extend based on customization needs. We work closely with you to establish a realistic timeline and keep you informed throughout the process.
-
Q: Do you provide long-term support and follow-up after delivering the iPSC-derived cells?
A: Yes, we offer comprehensive post-delivery support, including troubleshooting, protocol optimization, and technical consultation. We remain available to assist with any issues that may arise during your research, provide additional QC reports, and offer advice on downstream applications. Our goal is to be a long-term partner in your research, offering consistent support to maximize the success of your cell therapy development projects.
-
Q: How do you handle scalability for large-scale production of iPSC-derived cells?
A: We focus on developing scalable and robust protocols that transition smoothly from lab-scale to large-scale production. Our process optimization includes optimizing bioreactor systems, scaling differentiation protocols, and enhancing cell yields while maintaining quality. We also offer cryopreservation and banking services to facilitate long-term supply and consistent access to iPSC-derived cells for clinical or commercial purposes.
-
Q: Can you customize the iPSC-derived cells to match specific disease models?
A: Yes, our services are highly customizable to meet the unique requirements of your research. We can generate iPSCs from patient samples or genetically engineer them to carry specific disease-related mutations. Additionally, we offer tailored differentiation protocols to create cell types that best replicate the pathophysiology of your disease model, enabling more accurate and predictive studies.
Scientific Resources
Reference
-
Rami, Farzaneh, Halimeh Mollainezhad, and Mansoor Salehi. "Induced pluripotent stem cell as a new source for cancer immunotherapy." Genetics research international 2016.1 (2016): 3451807. Distributed under Open Access license CC BY 4.0, without modification.
For
Research Use Only. Not For Clinical Use.
 Fig.1 iPSC-derived cell therapy.1
Fig.1 iPSC-derived cell therapy.1