The blood-brain barrier (BBB) is formed by the brain capillary endothelium and excludes from the brain ∼100% of large-molecule neurotherapeutics and more than 98% of all small-molecule drugs. To overcome this hurdle, scientists from Creative Biolabs have developed single domain antibody technology that offers blood-brain barrier specific antibodies with implications for the development of biologic-based treatments for brain disorders.
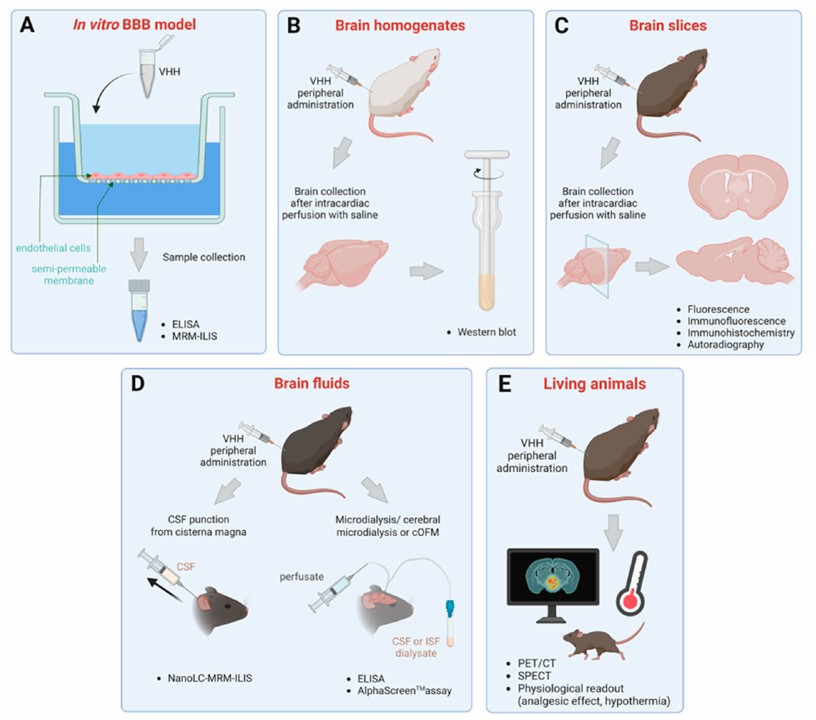 Fig. 1 A schematic representation of the methods previously reported to detect and quantify the passage of the BBB of single-domain antibodies. (Mireille Elodie Tsitokana, 2023)
Fig. 1 A schematic representation of the methods previously reported to detect and quantify the passage of the BBB of single-domain antibodies. (Mireille Elodie Tsitokana, 2023)
The BBB is a selective interface that restricts the movement of molecules between the bloodstream and the central nervous system. This barrier imposes a major challenge to the development of therapies for neurological diseases, large molecules in particular, due to the limited ability with which they can penetrate through the BBB. Single domain antibodies are considered very promising antigen-binding moieties for molecular imaging and therapeutic purposes because of their favorable pharmacological and pharmacokinetic properties. Single domain antibodies have been demonstrated to be able to cross the BBB without treatment, so they can be used as vectors to target drugs or therapeutic peptides into the brain.
Our technology also aims to target endogenous transport systems, including carrier-mediated transporters such as glucose and amino acid carriers, receptor-mediated transcytosis for insulin, transferrin, or low-density lipoproteins (LRP1), and the active efflux transporters such as p-glycoprotein.
Creative Biolabs is the recognized leader in single domain antibody production and single domain antibody library fields. We are specialized in generating single domain antibodies against any BBB targets.
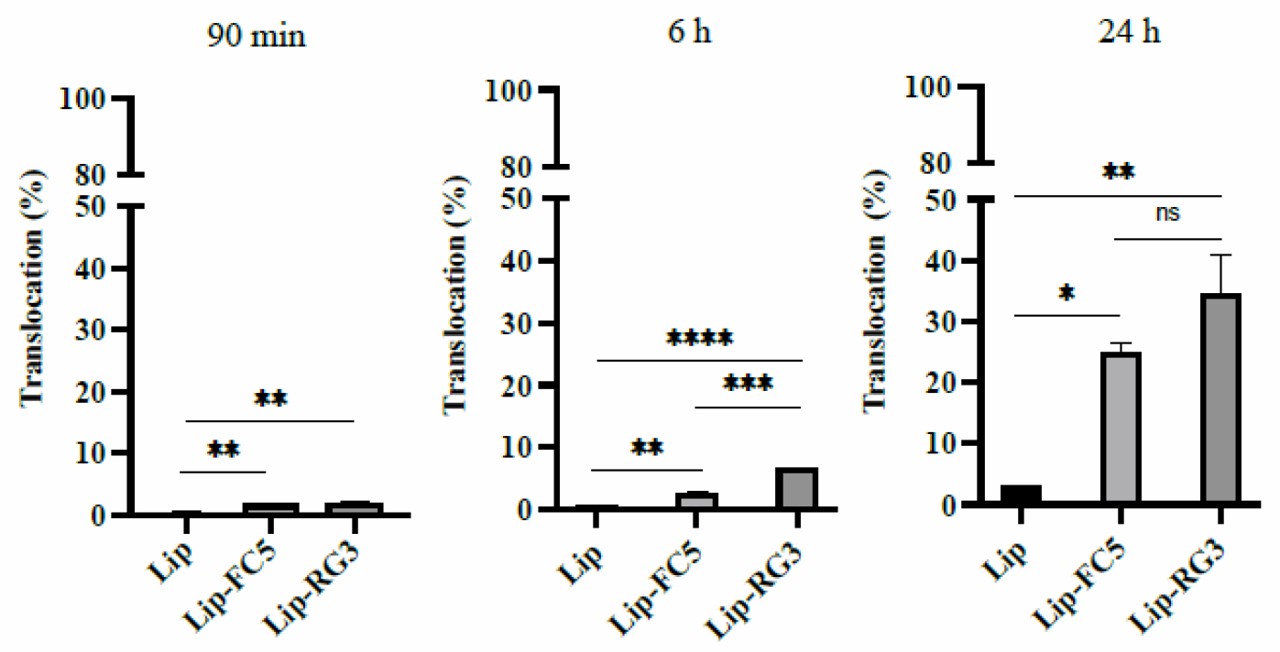 Fig. 2 Translocation of sdAbs functionalized liposomes in the in vitro BEB model. (Sandra Isabel Aguiar, 2021)
Fig. 2 Translocation of sdAbs functionalized liposomes in the in vitro BEB model. (Sandra Isabel Aguiar, 2021)
One of the difficulties in central nervous system (CNS) drug development is how to cross the blood-brain barrier. In this study, scientists described a cellular immune method in vivo to construct a rabbit-derived single-domain antibody library against the BBB endothelial cell receptors. After three rounds of screening, next-generation sequencing analysis, in vitro brain endothelial barrier (BEB) model screening and in vivo biological distribution studies, five potential sdAbs were identified, three of which reached more than 0.6 ID/g in the brain. In order to verify the concept of brain drug delivery, the most promising sdAb is conjugated to the surface of liposomes encapsulated with model drugs. The translocation efficiency and activity of conjugate liposomes were determined in bifunctional in vitro BEB-glioblastoma model. The results showed that sdAb-conjugated liposomes achieved effective BEB translocation and showed effective anti-tumor activity against LN229 glioblastoma cells without affecting the integrity of BEB.
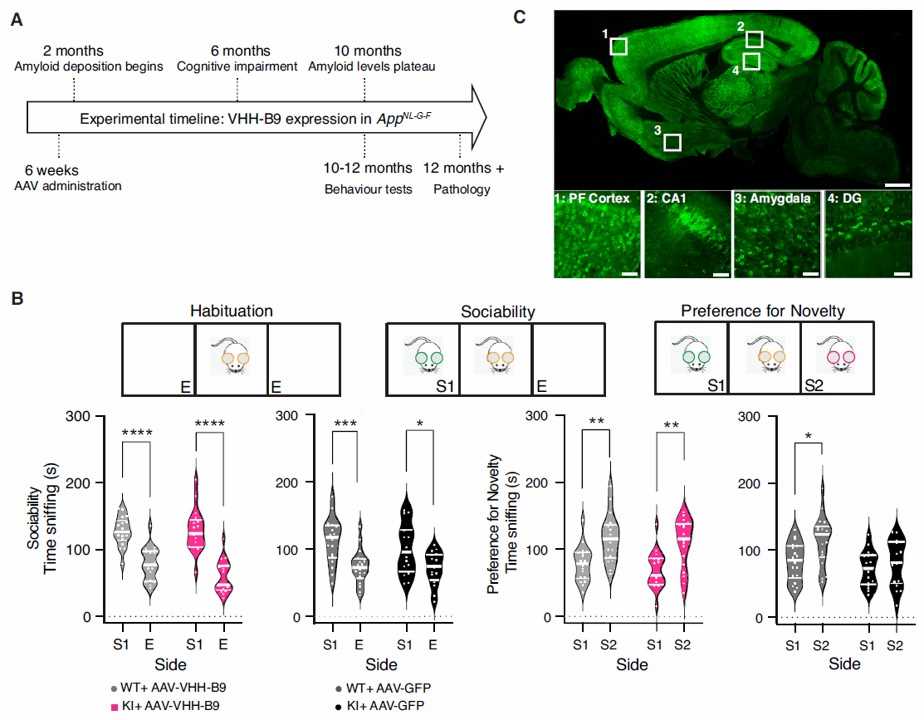 Fig. 3 Long-term BACE1 inhibition after AAV-VHH-B9 delivery reduces cognitive deficits in AppNL-G-F mice. (Marika Marino, 2022)
Fig. 3 Long-term BACE1 inhibition after AAV-VHH-B9 delivery reduces cognitive deficits in AppNL-G-F mice. (Marika Marino, 2022)
Single domain antibodies (sdAbs) have important biological value in the treatment of many diseases, including nervous system diseases. Here, scientists propose a gene transfer strategy based on the BBB cross adeno-associated virus (AAV) vector to transfer VHH directly into the CNS. Researchers explored the potential of AAV-transmitted VHH to inhibit BACE1, a clear target for Alzheimer's disease. Firstly, a group of VHHs for BACE1 were generated, one of which showed high selectivity for BACE1 and decreased the activity of BACE1 in vitro. Further studies have shown that in the AppNL-G-F Alzheimer's disease mouse model, a single whole body dose of AAV-VHH-B9 has a positive long-term (more than 12 months) effect on amyloid load, neuroinflammation, synaptic function, and cognitive ability. These results show that systematic applications using gene therapy vectors can effectively generate and deliver VHH targeting key CNS disease targets.
The smaller size of single-domain antibodies (~ 15 kDa) enables them to penetrate tissue more effectively and possibly through the BBB. SdAb's ability to traverse BBB is not inherent in all sdAbs, but can be designed or selected in a variety of ways. Common techniques include adding specific peptides that interact with BBB transport mechanisms through molecular engineering, or screening phage display libraries in vivo to identify variants that can effectively pass through the BBB.
Neurodegenerative diseases: diseases such as Alzheimer's disease, Parkinson's disease and Huntington's disease may benefit from sdAb because they bind to and neutralize the toxic proteins or aggregates believed to cause these diseases. For example, sdAb that can specifically target amyloid beta or tau proteins in Alzheimer's disease may help reduce its pathological effects.
Brain cancer: targeting tumor cells or tumor microenvironment with sdAb can provide a new way to directly deliver cytotoxic drugs or regulate immune response in the brain tumor site.
Acute neurological diseases: conditions such as stroke or traumatic brain injury may benefit from sdAb as they may be administered immediately after the event and reach the brain quickly to provide neuroprotection or help regulate inflammation.
Mental illness: there is interest in using sdAb to target pathological proteins or neurotransmitter receptors associated with mental disorders such as schizophrenia or depression.
BBB permeability: the first is to enhance the ability of sdAb to penetrate the BBB without compromising the integrity of the barrier. Strategies such as designing sdAb to interact with specific transporters or receptors on the BBB can improve its permeability, but precise molecular modification is needed.
Specificity and affinity: it is important to ensure that sdAb maintains high specificity and affinity for its target, as well as the ability to pass through BBB.
Production and stability: it is equally important to produce sdAb in a cost-effective and scalable manner and to maintain its stability and function during system administration. In addition, sdAb must remain stable in the bloodstream long enough to cross the blood-brain barrier and reach its target site at an effective concentration.
Safety and immunogenicity: evaluate the immunogenicity potential of sdAb, especially when modified to enhance BBB crossover.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
