Single domain antibodies are antibody fragments consisting of a single monomeric variable antibody domain. Like a whole antibody, it is able to bind selectively to a specific antigen. With beneficial pharmacologic and pharmacokinetic properties, they are ideally suited to targeting cellular antigens for molecular imaging or therapeutic purposes. However, because of their camelid or nonhuman origin, the possible immunogenicity of single domain antibodies when used in the clinic is a concern.
Creative Biolabs has extensive experience in generating human single domain antibodies and humanizing single domain antibodies. We have developed synthetic human single domain antibody phage display libraries using humanized single domain antibody scaffolds. Human single domain antibodies against all targets can be rapidly isolated from these libraries. In addition, by immunizing a unique strain of transgenic mice available from a partner that harbors human single domain antibody gene repertoires, Creative Biolabs is able to produce immune human single domain antibody libraries that can produce high affinity human single domain antibodies against the immunogens.
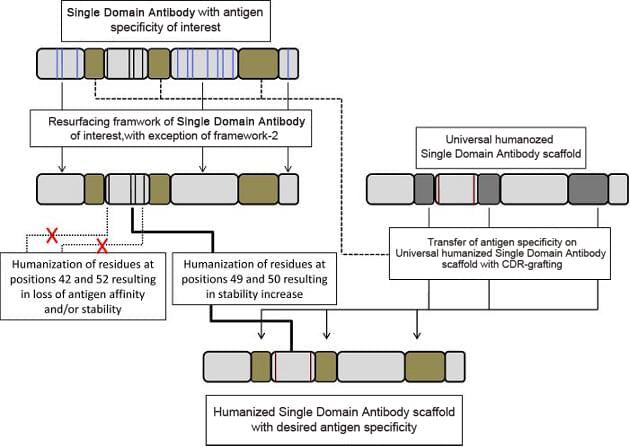
Single domain antibody humanization services are also available at Creative Biolabs. In the first straightforward approach that employs soluble, stable, well expressed universal humanized single domain antibody scaffolds, CDR regions of a parental single domain antibody can be directly grafted with the antigen-specificity and affinity of the parental antibody transferred. Regular CDR grafting plus the back-mutation method is also frequently used to humanize single domain antibodies at Creative Biolabs.
A humanized single domain antibody is an engineering version of sdAb, modified to improve its compatibility with the human immune system. Humanization involves modifying the amino acid sequences of sdAb to make them more similar to human antibody sequences, thereby reducing their immunogenicity. This process is important for therapeutic applications because it minimizes the risk of adverse immune reactions, such as the production of anti-drug antibodies (ADAs), that neutralize therapeutic effects or cause other complications.
Oncology: sdAbs is small in size and can penetrate tumors more effectively, thus accurately targeting cancer cells or the tumor microenvironment.
Infectious diseases: sdAbs can bind to conserved regions of viruses or bacteria, making them excellent candidates for neutralizing sources of infection.
Autoimmune and inflammatory diseases: humanized sdAb can be designed to block cytokines or cell surface receptors involved in autoimmune and inflammatory processes.
Neurological diseases: sdAbs has the potential to cross the blood-brain barrier and can be used to target pathological proteins related to diseases such as Alzheimer's or Parkinson's disease.
Selection of targets and initial antibody production.
Sequencing and humanization.
Synthesis and expression of humanized antibodies.
Functional and immunogenicity tests.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
