As a recognized leader in the field of phage display and antibody engineering, Creative Biolabs is experienced in conducting affinity maturation projects, with affinity maturation of single domain antibodies in particular.
Although single domain antibodies isolated from antibody libraries derived from gene synthesis and immunized or naïve animals generally exhibit reasonable affinity, it is often desirable to further increase their affinity. Quantitative library sorting by phage display or yeast surface display is used to improve the affinity of single domain antibodies. In particular, we have developed a proprietary DNA mutagenesis technique that is able to create a huge number (e.g., 1010) of variants of the parental antibody with defined positions mutated. In combination with our first-class phage display antibody library construction and screening technologies, 10–100 fold increase in affinity for parental single domain antibodies of low nM affinity is frequently achieved. This is often possible for other companies for antibodies of 100 nM affinity or worse.
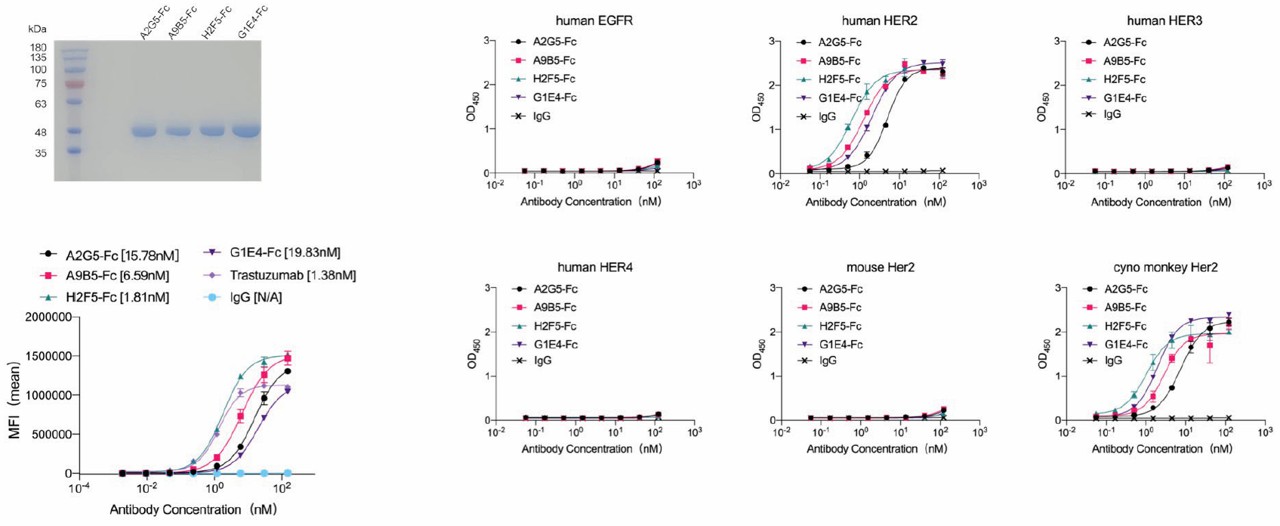 Fig. 1 Affinity maturation of HER2-targeted sdAbs.1
Fig. 1 Affinity maturation of HER2-targeted sdAbs.1
Human epithelial growth factor receptor 2 (HER2) plays a carcinogenic role in many tumors. In this study, using the synthetic single-domain antibody library and affinity maturation, four high affinity and specific anti-HER2 single-domain antibodies were identified. The chimeric HER2-ECD protein showed that these single domain antibodies could recognize three different HER2-ECD domains. In addition, to further study the potential therapeutic benefits, scientists constructed VHH-Fc fuses and found that they can promote internalization and show moderate growth inhibition. Compared with the single traditional monoclonal antibody combination, the VHH-Fc combination or its combination with traditional monoclonal antibodies showed greater or equivalent anti-tumor activity in ligand-independent and ligand-driven tumors. This result provides anti-HER2 sdAbs with high affinity and non-overlapping epitope recognition.
The affinity maturation of single domain antibodies is usually based on molecular evolution techniques, such as phage display or yeast display. First of all, a single domain antibody library containing random or semi-random mutations is constructed. Then, the antibody variants with higher affinity were selected by combining with the fixed antigen. In addition, computational biology methods can also be used to predict possible mutation sites so as to design antibody variants with potentially higher affinity before the experiment. These methods help to accelerate the development and optimization of antibodies and make them more effective and specific in clinical applications.
One of the main challenges of single-domain antibody affinity maturation is maintaining the stability and solubility of antibodies. In the process of affinity-enhanced mutation, it is possible to introduce changes that are disadvantageous to the structure of the antibody, which may lead to antibody aggregation or degradation. In addition, the improvement of affinity may sometimes affect the specificity of antibodies, that is, high-affinity antibodies may cross-react with non-target antigens. Therefore, the process of affinity maturation requires fine balance and extensive functional testing to ensure that the performance of the antibody will not be impaired by the improvement of affinity.
Commonly used techniques include directed evolution, mutation site analysis, and computer-aided design. Directed evolution technology through the construction of a large-scale variation library, and the use of high-throughput screening techniques such as phage display or cell surface display to find more affinity variants. Mutation site analysis is based on the existing structural and functional data and selects specific amino acid residues for mutation. Computer-aided design uses bioinformatics tools to predict the mutation points that may enhance affinity by simulating the interaction between single-domain antibodies and their antigens.
First of all, biophysical methods such as surface plasmon resonance (SPR), enzyme-linked immunosorbent assay (ELISA), or biolayer interferometry (BLI) can be used to determine the specific values of affinity, which can provide detailed information about the binding strength and kinetics of antibodies to antigens. Secondly, cell function tests, such as inhibition tests or cytotoxicity tests, were carried out to verify whether the affinity-enhanced antibody maintained or improved its biological function. In addition, the expression level, stability, and solubility of the antibody need to be evaluated to ensure that the overall quality of the antibody meets the requirements of follow-up applications. These comprehensive assessments can ensure the success of the affinity maturation process and the functionality of the antibody product.
In the field of clinical medicine, these high-affinity single-domain antibodies can be used in targeted drug delivery systems to improve drug targeting and reduce side effects. They are also commonly used in cancer treatment and can effectively guide the immune system to attack cancer cells by accurately identifying and binding specific antigens on the surface of tumor cells. In addition, because of the high stability and easy modification of single-domain antibodies, they can also be used to develop rapid diagnostic tests, especially in cases where rapid diagnosis of infectious diseases or other emergency medical conditions is required. In addition, single-domain antibodies with mature affinity are widely used in the field of scientific research for the research of basic biological processes and the development of biomarkers.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
