While aTM and aLN hydrogel scaffolds show promise in mimicking the ECM of secondary lymphoid organs for antigen-specific T cell activation, they are limited by reproducibility, structural integrity, and in vivo degradation. To address these limitations, scientists are investigating novel polymer-based scaffolds with tailored architectures, tunable flexibility, and precisely controlled surface modifications. These polymeric materials offer potential advantages in terms of stability, controlled degradation, and tailored interactions with immune cells.
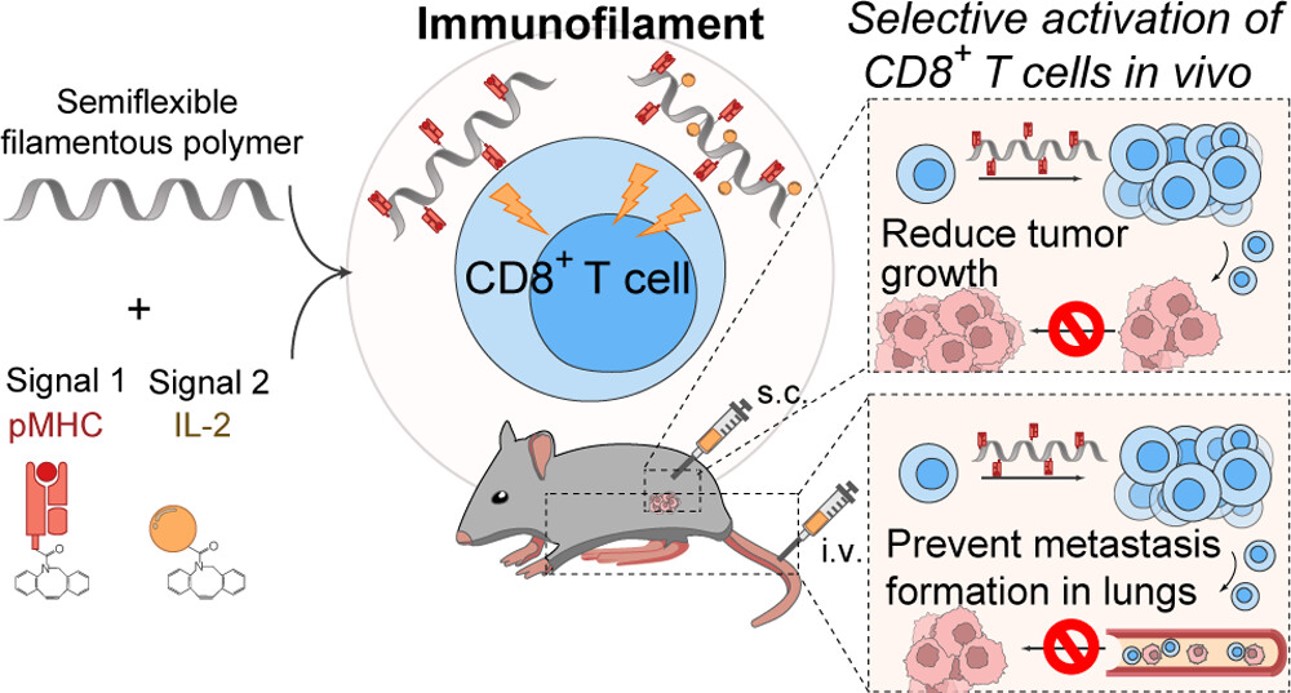 Fig.1 Polymeric-based artificial T cell stimulating platform exhibits the ability to activate T cell.1
Fig.1 Polymeric-based artificial T cell stimulating platform exhibits the ability to activate T cell.1
To optimize the efficacy of the polymeric-based artificial T cell-stimulating platform, Creative Biolabs integrates alternative polymeric materials to develop an artificial T cell-stimulating platform with improved reproducibility, structural integrity, and controlled degradation compared to traditional hydrogel-based scaffolds. Our development services encompass:
In addition, Creative Biolabs offers comprehensive characterization services for our artificial T cell-stimulating platform, covering the entire development process from in vitro assessment of the stimulating platform's properties to in vivo evaluation of its efficacy. We leverage cutting-edge technology and rigorous quality assurance to deliver consistent and dependable results throughout the process. Throughout the process, we work closely with our clients, providing timely updates and adhering to agreed-upon timelines, to accelerate the development of more effective and durable cancer T cell therapies. This comprehensive approach empowers researchers with the data and insights necessary to translate their discoveries into impactful clinical applications.
Summary: This study develops polymeric based-nanosized immunofilaments to mimic the natural T cell activation. Results reveal that the nanosized immunofilaments are able to directly activate T cells in vivo, inhibiting tumor growth and metastasis. Moreover, the immunofilaments are also able to effectively activate and expand tumor-specific T cells in vitro and in vivo, leading to a significant reduction in tumor growth and metastasis in mice. This polymeric-based approach exhibits the potential to improve cancer immunotherapy by enhancing the efficacy of T cell-based therapies.
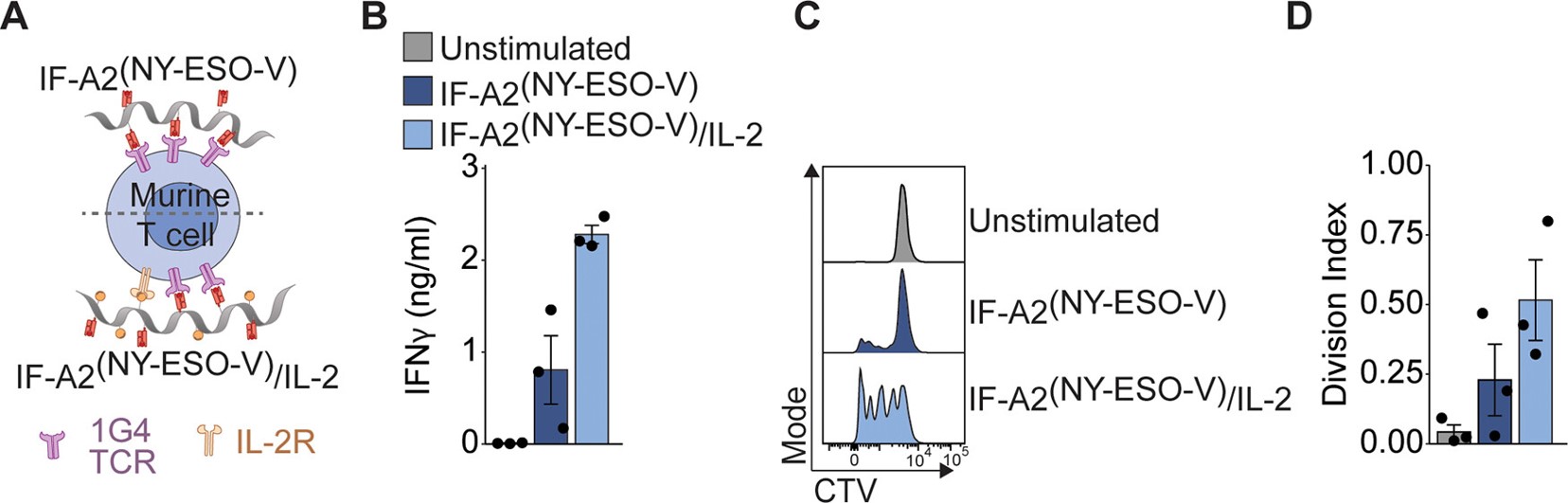 Fig.2 Engineered immunofilaments successfully activate and expand tumor-specific CD8+ T cells in mice.1
Fig.2 Engineered immunofilaments successfully activate and expand tumor-specific CD8+ T cells in mice.1
Beyond our advanced polymeric artificial T cell stimulation platforms, Creative Biolabs leverages a diverse portfolio of cutting-edge materials to create highly effective and customizable solutions. This breadth of expertise allows us to tailor each platform to your specific research requirements, providing a wide array of options for optimized T cell stimulation. Our proficiency in multiple technologies ensures that we can deliver the ideal solution for your unique project goals, maximizing the potential for success in your T cell research.
If you are interested in developing a high-performance, custom-designed polymeric platform for artificial T cell stimulation, please contact us to get more information.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION