Product List Background
Background
MUC1 is a membrane-bound protein that forms protective mucous barriers on epithelial surfaces through O-glycosylation. It functions in intracellular signaling and cell adhesion, predominantly expressed on the apical surface of epithelial cells lining various mucosal tissues. Proteolytic cleavage yields alpha and beta subunits, forming a heterodimer. The N-terminal alpha subunit mediates cell adhesion, while the C-terminal beta subunit participates in cell signaling via phosphorylation and protein interactions. Abnormalities such as overexpression, altered intracellular localization, and glycosylation changes in MUC1 are linked to carcinomas. Associated diseases include autosomal dominant and so on.
Its Gene ID: 4582, UniProtKB ID: P15941, and OMIM ID: 158340.
MUC1 Involved Signaling Pathways
MUC1 has promoted radioresistance via JAK2/STAT3 signaling activation in hepatocellular carcinoma. In cervical cancer, MUC1 enhances EGFR nuclear translocation. In contrast, in colorectal cancer, MUC13 activates the NF-κB pathway to protect cells from death, and MUC1 blocks c-Abl nuclear targeting in response to DNA damage. These studies suggest mucin glycoproteins inhibit apoptosis through various pathways, fostering an apoptosis-resistant phenotype in cancer cells. MUC1-C engages with EGFR, ErbB2, and additional receptor tyrosine kinases, initiating the PI3K→AKT and MEK→ERK signaling pathways. Additionally, MUC1-C translocates to the nucleus and activates the Wnt/β-catenin, STAT, and NF-κB RelA signaling pathways.
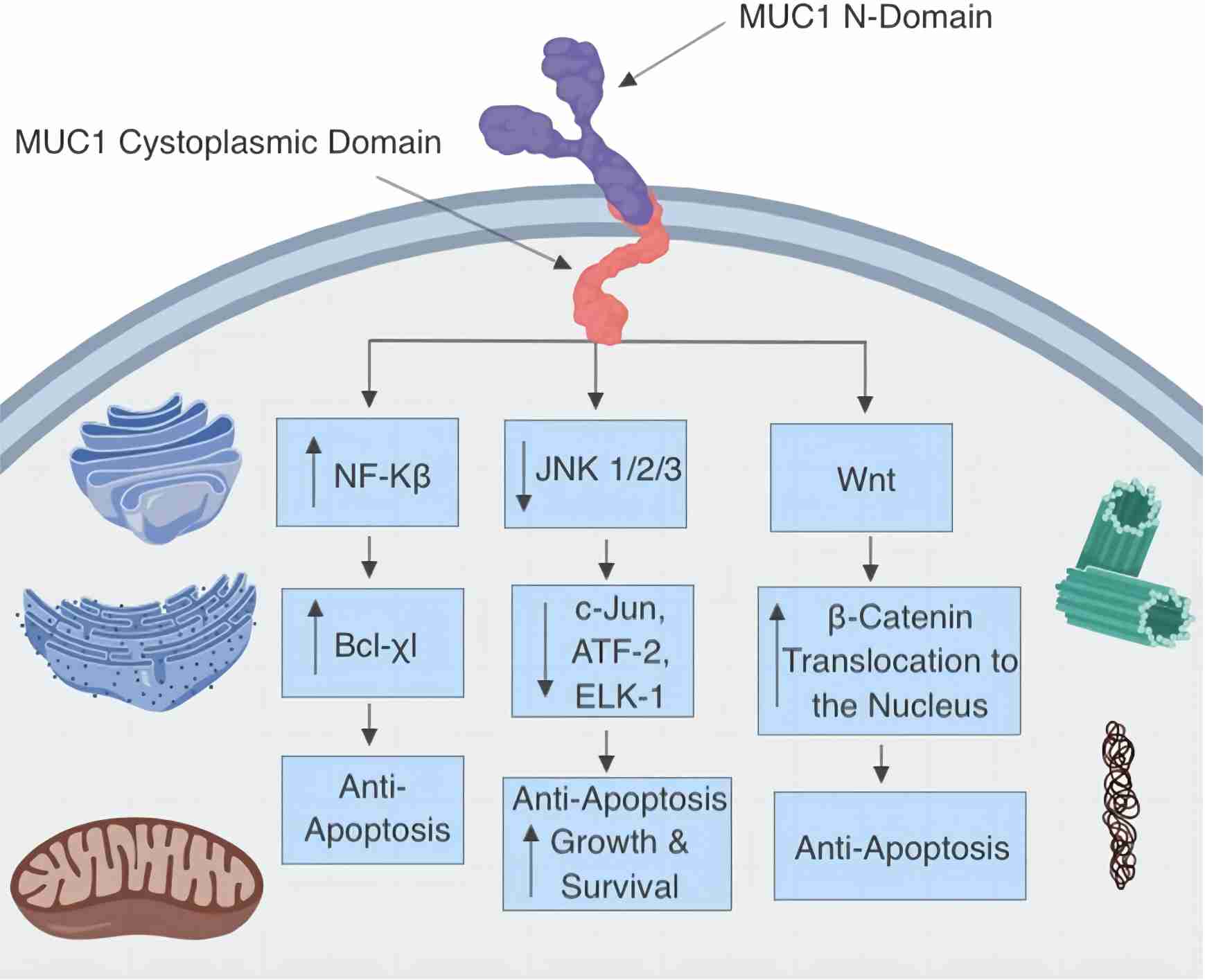 Fig.1 Mucin glycoprotein-targeted signaling pathways: MUC1 interaction and apoptosis inhibition.1
Fig.1 Mucin glycoprotein-targeted signaling pathways: MUC1 interaction and apoptosis inhibition.1
Applications of MUC1-Related Products
-
Anti-MUC1 Aptamer-Nanoparticle Conjugates for Targeted Drug Delivery in Tumors
The MUC1 protein, exhibiting heightened expression in most adenocarcinomas, is an appealing target for delivering anticancer drugs. An identified aptamer targeting the MUC1 protein has been employed within a nanoparticle drug delivery system. Polymeric nanoparticles encapsulating paclitaxel (PTX) are synthesized via an emulsion/evaporation method, with the attachment of MUC1 aptamers to the nanoparticle surface facilitated through a DNA-based linker. Flow cytometry has demonstrated that the MUC1 aptamer increases nanoparticle uptake into target cells. Furthermore, PTX-loaded aptamer-functionalized nanoparticles (Apt-NPs) improve drug delivery and cytotoxicity against cancer cells in vitro, outperforming nanoparticles without targeting ligands. This novel aptamer-nanoparticle bioconjugate suggests potential applications in targeted drug delivery for MUC1-overexpressing tumors.
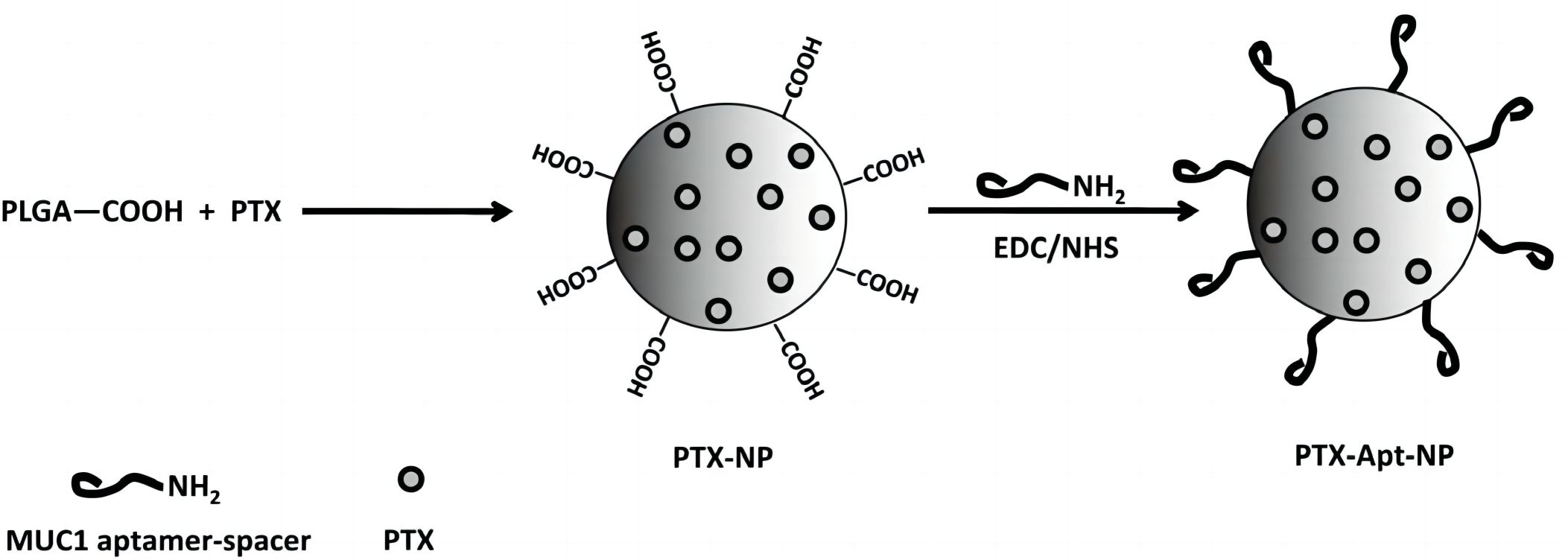 Fig.2 Schmatic diagram of anti-MUC1 aptamer-nanoparticle structure.2
Fig.2 Schmatic diagram of anti-MUC1 aptamer-nanoparticle structure.2
-
New Therapeutic Approach Using Bispecific Aptamers Targeting MUC1 & CD16
A novel approach in cancer immunotherapy involves utilizing a dual-targeting agent capable of binding both tumor-specific markers and immune cells to facilitate lymphocyte recruitment to tumor sites and bolster anticancer immune responses. MUC1, highly expressed in nearly all adenocarcinomas, represents a critical target for this strategy. CD16 serves as a key immunocyte marker. Researchers developed the first bispecific aptamer (BBiApt) designed to target MUC1 and CD16 concurrently. This aptamer comprised two MUC1-specific and two CD16-specific aptamers connected by three DNA spacers. Compared to monovalent aptamers, BBiApt demonstrates enhanced affinity for MUC1-positive tumor cells and CD16-positive immunocytes. Competitive binding assays confirm that BBiApt and monovalent aptamers compete for the same binding sites on target cells. Furthermore, BBiApt facilitates increased recruitment of CD16-positive immunocytes around MUC1-positive tumor cells and augments immune-mediated cytotoxicity against these tumors in vitro. These findings underscore the potential of bispecific aptamers, alongside bispecific antibodies, as a promising strategy to selectively boost antitumor immune responses against MUC1-expressing cancers.
Creative Biolabs provides an extensive range of products related to MUC1, encompassing assay kits and aptamers designed specifically for MUC1 detection. Moreover, we offer customized MUC1-specific solutions, including bespoke bispecific antibodies crafted to address individualized needs and specifications.
References
-
Reynolds, Ian S., et al. "Mucin glycoproteins block apoptosis; promote invasion, proliferation, and migration; and cause chemoresistance through diverse pathways in epithelial cancers." Cancer and Metastasis Reviews 38 (2019): 237-257.
-
Yu, Chenchen, et al. "Novel aptamer-nanoparticle bioconjugates enhance delivery of anticancer drug to MUC1-positive cancer cells in vitro." PloS one 6.9 (2011): e24077.


 Datasheet
Datasheet Fig.1 Mucin glycoprotein-targeted signaling pathways: MUC1 interaction and apoptosis inhibition.1
Fig.1 Mucin glycoprotein-targeted signaling pathways: MUC1 interaction and apoptosis inhibition.1
 Fig.2 Schmatic diagram of anti-MUC1 aptamer-nanoparticle structure.2
Fig.2 Schmatic diagram of anti-MUC1 aptamer-nanoparticle structure.2
