Product List Background
Background
Creative Biolabs provides an extensive selection of top-notch anti-S. typhimurium OMP aptamers to support researchers in enhancing their experiments and studies. We uphold our standards for excellence and are dedicated to assisting in your research in biology.
Introduction
Salmonella typhimurium outer membrane protein (OMP), abbreviated as S. typhimurium OMP, is a pure recombinant antigen produced in E. coli cells for the development of immunoassays and various other uses. Outer membrane proteins (OMPs) are a large family of β-barrel proteins found in membranes of bacteria and eukaryotes. OMPs have a significant impact on adjusting to environmental circumstances, mobility, adhesion, and colonization of host cells. They are also responsible for transporting nutrients and ions across the cell membrane. Due to the ease of expression and refolding, as well as simple methods for monitoring the extent of folding, OMPs are excellent model systems for studying the membrane environment and how other biological factors affect the folding pathway.
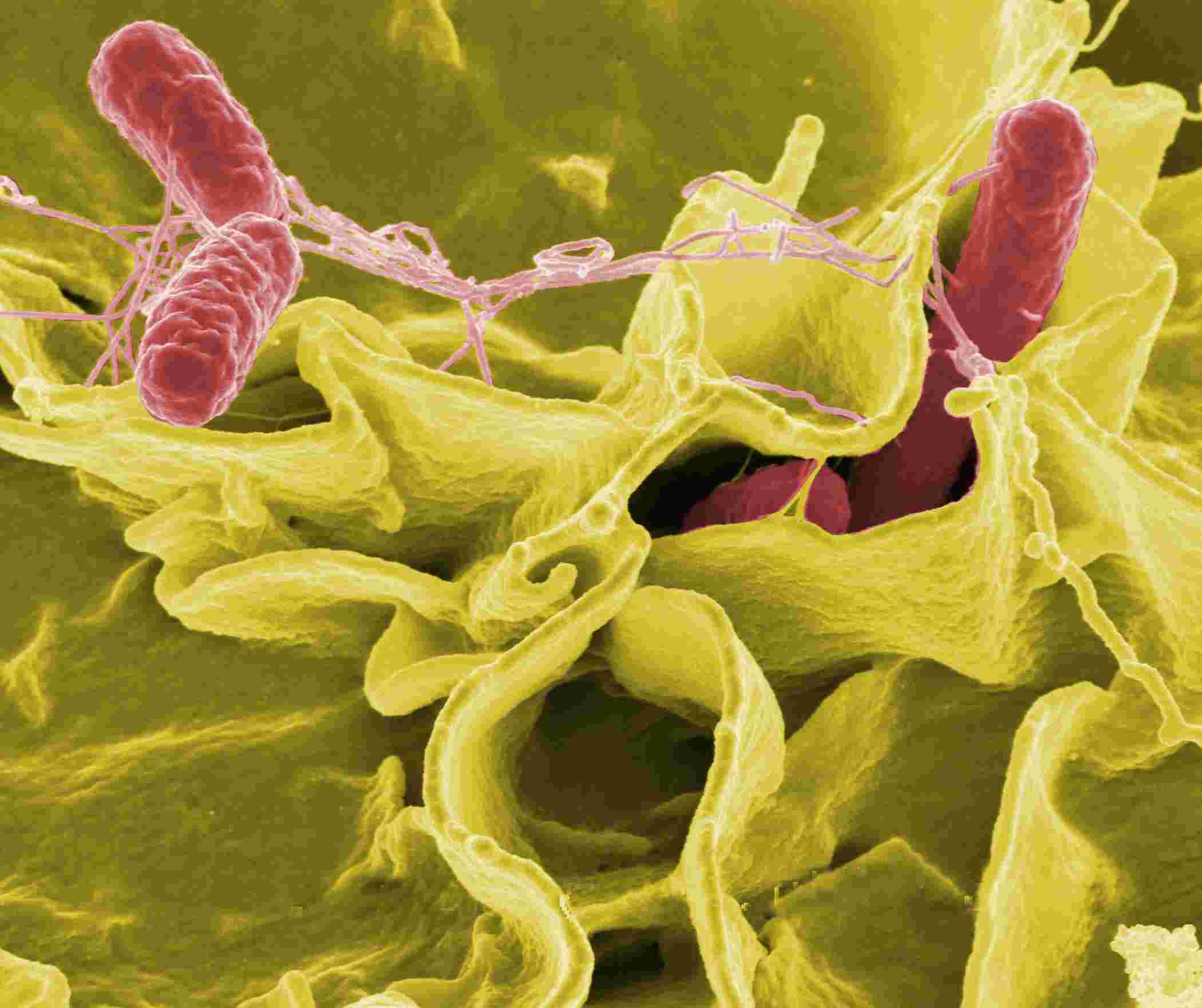 Fig.1 S. typhimurium (red).1
Fig.1 S. typhimurium (red).1
S. typhimurium is an important genus of the Enterobacteriaceae family that can cause diarrhea, gastroenteritis, typhoid fever, paratyphoid fever, sepsis, and other clinical syndromes of varying severity. S. typhimurium is the most common pathogen that causes typhoid fever and belongs to the serotype of Salmonella enteric. Brown lipoprotein, porins (ompC, ompF, ompD, phoE, etc.), and heat-modifiable protein (omp A) are among the OMPs of S. typhimurium.
Main Types of OMPs
OMPs are a general term for all proteins present in and related to the bacterial outer membrane, containing many types of proteins. According to the number of proteins in the outer membrane and the importance of their functions, they can be divided into significant OMPs and minor OMPs. The major OMPs can be divided into porins, ompA proteins, and lipoproteins.
-
Porins are a subclass of OMPs that form tiny channels in membranes, allowing passive transport of hydrophilic compounds, which helps regulate cell permeability and increase antibiotic resistance.
-
The ompA proteins belong to a cluster of genetically similar porins that are heat-modifiable and located on the surface, found in high quantities in the outer membrane of Gram-negative bacteria. OmpA proteins play crucial roles in many processes, such as bacterial attachment, penetration, evasion of host immune responses, survival inside cells, and triggering the production of pro-inflammatory cytokines. Members of the ompA protein family can be targeted by the immune system, and their ability to trigger an immune response is linked to the surface-exposed loops of the molecules.
-
Lipoproteins are structural proteins of the bacterial outer membrane, with a small molecular weight of about 7200 d. About 1/3 of it is called bound lipoprotein, which is covalently cross-linked with the cell wall acid of peptidoglycan through the -NH2 residue of the C-terminal lysine. The remaining 2/3 are free lipoproteins. Although strains with mutations in the lipoprotein structural gene can allow small molecular weight hydrophilic molecules to pass through the outer membrane, the cell wall of this mutant strain is unstable, and the outer membrane components can be released in the form of blisters. Therefore, in addition to being a structural protein, lipoprotein also plays a role in stabilizing the bacterial outer membrane-peptidoglycan complex.
Creative Biolabs provides various kinds of S. typhimurium OMP related products to fulfill your diverse experimental requirements.
Reference
-
From Wikipedia: NIAID, Public Domain, https://commons.wikimedia.org/wiki/File:SalmonellaNIAID.jpg


 Datasheet
Datasheet Fig.1 S. typhimurium (red).1
Fig.1 S. typhimurium (red).1
