The motor system is a highly complex system that controls body movement. It can be divided into 1) the peripheral apparatus, which consists of the anterior horn cell and its peripheral axon, the neuromuscular junction, and muscle, and 2) the more complex central apparatus, which includes the descending tracts involved in control and the systems involved in initiating and regulating movement (the basal ganglia and cerebellum). Motor system is responsible for conscious design and execution of motor planning as well as for numerous unconscious processes. The muscles are the last link of this very long and complex pathway. Dysfunction of any component of this system will lead to variable degrees of compromise in the ability to perform motor acts, including total loss of function.
The motor neuron diseases (MNDs) are a group of progressive neurological disorders that destroy motor neurons, the cells that control skeletal muscle activity. This group includes diseases such as amyotrophic lateral sclerosis (ALS), progressive bulbar palsy (PBP), primary lateral sclerosis (PLS), progressive muscular atrophy (PMA), spinal muscular atrophy (SMA), Kennedy's disease, and Parkinson's disease (PD). Currently, there is no cure or standard treatment for MNDs. Symptomatic and supportive treatment can help people affected by MNDs be more comfortable while maintaining their quality of life. The stem cells specific to different diseases are necessary to advance disease-specific therapy. Creative Biolabs provides the disease-specific stem cell therapy development service for our customers upon different demands.
Induced pluripotent stem cells (iPSCs) can be induced to neural progenitor cells (NPCs) that possess multi-potency to multiple neural lineages. With additional induction, NPCs can give rise to neurons, astrocytes and oligodendrocytes. Microglia can be induced from myeloid progenitor cells (MPCs) generated from iPSCs. Each neural cell type can be used to model relevant neurological diseases. These iPSC-based artificial model systems are of paramount importance in searching for adequate therapeutic agents, as well as for a deep understanding of the MND pathogenesis.
Combined with iPSC technology, neural stem cells (NSCs) can be generated from iPSCs through mimicking in vivo developmental environment and applied to neurological disease modeling. Scientists found that synergistic inhibition of glycogen synthase kinase (GSK3), transforming growth factor β (TGFβ) and NOTCH pathways using small molecules can efficiently induce human ESCs to NSCs within one week. These NSCs express characteristic markers, such as N-Cadherin, SOX2 and NESTIN. Using iPSC-derived NSCs, a wide range of MNDs have been studied.
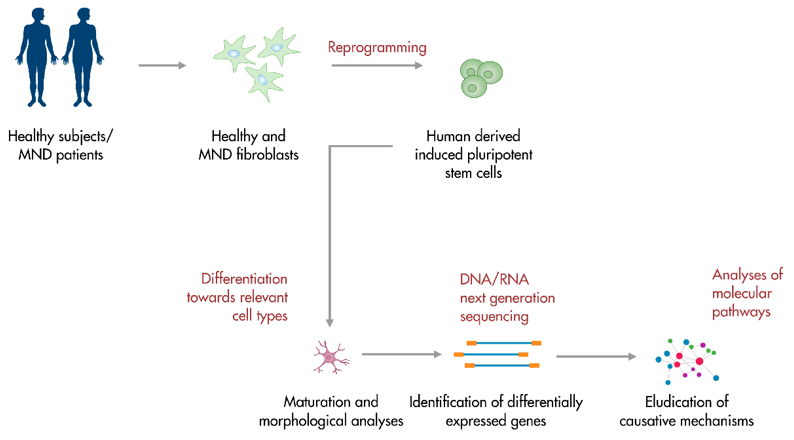 Fig.1 iPSC-based platforms for motor neuron disease modeling.1, 3
Fig.1 iPSC-based platforms for motor neuron disease modeling.1, 3
iPSC-derived neurons have attracted great interest for modeling neurodegenerative diseases. Alzheimer's disease (AD), PD, and ALS are three hot topics for disease modeling using iPSC-derived neurons. For instance, an electrophysiological study of iPSC-derived motor neurons with mutations in the SOD1 gene, as well as in C9ORF72 and FUS, revealed the hyperexcitability of their membranes that may be the main element of the ALS pathogenesis, leading to the death of motor neurons.
iPSC-derived astrocytes have also been used to model AD and ALS. AD iPSC-derived astrocytes were found to exhibit distinct morphology from normal astrocytes with less complexity and aberrant marker localization. Since astrocytes are highly heterogeneous, the development of subtype-specific astrocytes would allow more accurate disease modeling.
Unlike other neural cell types that have been generated from iPSCs using a variety of protocols, derivation of microglia from iPSCs has become the focus of great interest just in recent years. Scientists developed microglia-like cells from human iPSCs through inducing myeloid progenitors from embryo body (EB) and these cells express microglia-specific transcriptional factor PU.1 and surface markers IBA1, CD11B and CD45. Furthermore, a new protocol has been published and it gives rise to microglia that express a comprehensive set of lineage-defined markers, including HLA-DR, CD45, TREM-2 and CX3CR1.
iPSCs can be used not only to explore alternative modes of therapy, but also to search for new compounds as potential drugs for MNDs and spinal cord injuries. iPSCs can be re-differentiated into specific neural cell types and employed in assay development, drug screens, lead development, new drugs and clinical trials, leading to new therapies for MNDs.
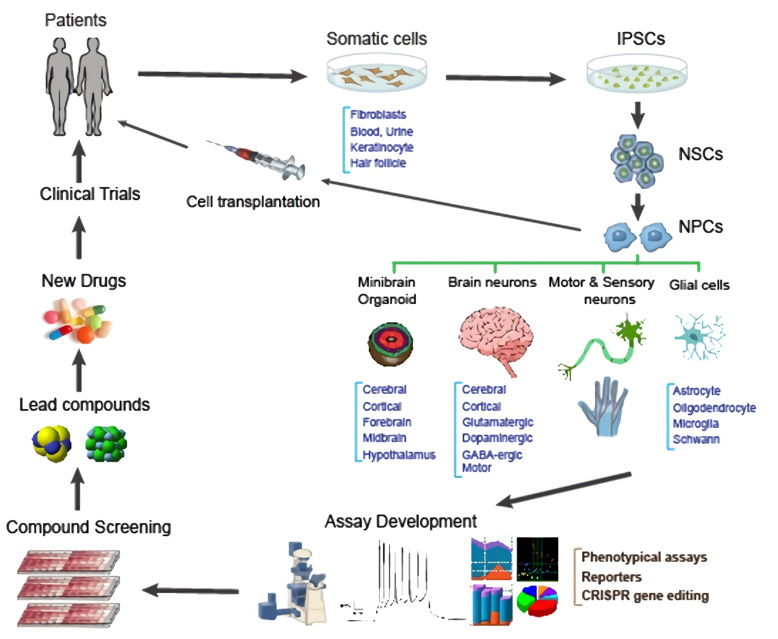 Fig.2 Applications of iPSCs in neural drug discovery.2, 3
Fig.2 Applications of iPSCs in neural drug discovery.2, 3
With extensive expertise in iPSC differentiation, and stem cell facilitated drug discovery, Creative Biolabs is competent to provide MNDs model construction and therapy development services for global clients. Please contact us for detailed information.
References
For Research Use Only. Not For Clinical Use.