Respiratory system diseases are common and major ailments that affect the lungs and other parts of the respiratory system. Diseases of the respiratory system impose an immense worldwide health burden and have been a leading cause of morbidity, mortality and disability. There are five most-common conditions that primarily contribute to the global burden of respiratory diseases: asthma, chronic obstructive pulmonary disease (COPD), acute respiratory infections, tuberculosis, and lung cancer. Globally, hundreds of millions of people suffer and four million people die prematurely from respiratory diseases each year.
COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a respiratory illness that primarily targets the lungs and causes lasting lung damage. COVID-19 may cause a range of breathing problems, from mild to critical.
COVID-19 may cause pneumonia. Air sacs in the lungs fill with fluid, limiting their ability to take in oxygen and causing shortness of breath, cough and other symptoms. COVID-19 associated pneumonia may be severe, with prolonged lung injury and breathing difficulties.
As COVID-19 pneumonia progresses, acute respiratory distress syndrome (ARDS) may occur accompanied by the symptom that more of the air sacs become filled with fluid leaking from the tiny blood vessels in the lungs. ARDS can be fatal. Even survive and recover from COVID-19, patients have lasting pulmonary scarring.
Sepsis occurs when SARS-CoV-2 infection reaches and spreads through the bloodstream, causing tissue damage everywhere it goes. The cooperation between lungs, heart and other organs falls apart, and entire organ systems start to shut down. Sepsis causes lasting damage to the lungs and other organs.
COVID-19 patients may be more vulnerable to infection with another bacterium or virus, and suffer a superinfection. More infection can result in additional lung damage.
With the constant increase of respiratory diseases, improving in vitro lung models is essential to maximumly reproduce the complex pulmonary architecture and function, responsible for oxygen uptake and carbon dioxide clearance. Recent development in the fields of iPSCs and organoids technologies has provided new insights to better understand pulmonary diseases and to find new therapeutic perspectives.
Patient-specific iPSCs hold great promise, both as a biological tool to uncover the pathophysiology of respiratory disease by creating relevant human cell models and as a source of cells for cell-based therapeutic discovery and drug screening. Tamò, et al have established an AEC II cell line (LL-iPSC-AEC II) derived from iPSCs to study alveolar epithelial cell biology, for instance, in the context of lung injury, fibrosis, and repair. LL-iPSC-AEC II cell lines displayed morphological characteristics of AEC II (cobblestone monolayer, lamellar bodies, and microvilli); expressed AEC type II proteins (cytokeratin and surfactant protein C); exhibited functional properties of AEC II (an increase of transepithelial electrical resistance over time, secretion of inflammatory mediators in biologically relevant quantities (IL-6 and IL-8)); showed efficiency in vitro alveolar epithelial wound repair. Therefore, iPSC-derived respiratory system models can be used as a novel cellular model to study pulmonary cell biology and reveal disease mechanisms and develop respiratory system drug.
Organoids are self-organizing 3D structures derived from stem cells that are supported by an extracellular matrix and contain multiple cell types whose spatial arrangement and interactions mimic the native organ. In other words, organoids recapitulate essential aspects of organ structure and function. Respiratory system organoids have been generated from adult lung stem cells and human iPSCs to model human lung development, and can serve as a platform for investigating the function of lung-related genes and signaling pathways and discovering novel therapeutic strategies and drugs. Human airway organoids have been developed as a model to study SARS-CoV-2 replication kinetics, tropism, infectivity, and host response to help fight the current pandemic.
Patient-specific iPSCs and organoids may serve as an autologous cell and model source for respiratory disease research and therapeutic drug discovery. With a decade of experience in stem cell therapy development, Creative Biolabs provides high-quality iPSC reprogramming & differentiation & characterization and organoids development services to our customers all over the world. Our customized services cover the entire development process of iPSC and organoids to best suit your technical, program, and budget requirements. We will be your reliable partner in stem cell basic research and cell therapy.
Our iPSC platform is designed to advance research in respiratory system diseases, including but not limited to asthma, COPD, pulmonary fibrosis, and viral infections such as COVID-19. The platform provides researchers with the tools and resources needed to explore the biology of the respiratory system, screen potential drug candidates, and develop novel therapeutic strategies. Key components of our service include:
Our iPSC platform for respiratory system research is a powerful resource for advancing the understanding of lung diseases and developing novel therapeutic strategies. By providing access to well-characterized iPSC lines and comprehensive differentiation protocols, we empower researchers to make significant contributions to respiratory health.
An iPSC platform specifically for respiratory system research can offer numerous advantages for a biotechnology company. Our iPSC platform for respiratory system research is a cutting-edge tool that empowers researchers to conduct high-quality studies on respiratory cell function, disease mechanisms, drug discovery, and personalized medicine. Designed for research use only, the platform provides the following key advantages:
Below are the findings presented in the article related to iPSC research platform for respiratory system.
Adam Mitchell et al. developed a method for generating lung organoids by co-culturing defined endodermal and mesodermal progenitor cells. Endodermal and mesodermal progenitor cells were differentiated from iPSCs, then combined in 3D Matrigel hydrogels and differentiated for a further 14 days to generate lung organoids. The organoids expressed a range of lung cell markers such as SPA, SPB, SPC, AQP5, and T1α. In addition, the organoids expressed ACE2, which binds to SARS-CoV-2 spiny proteins, demonstrating the physiological relevance of the resulting organoids. This study utilizes a polyembryonic approach for the rapid production of lung organoids, which can be used to study respiratory-related human diseases.
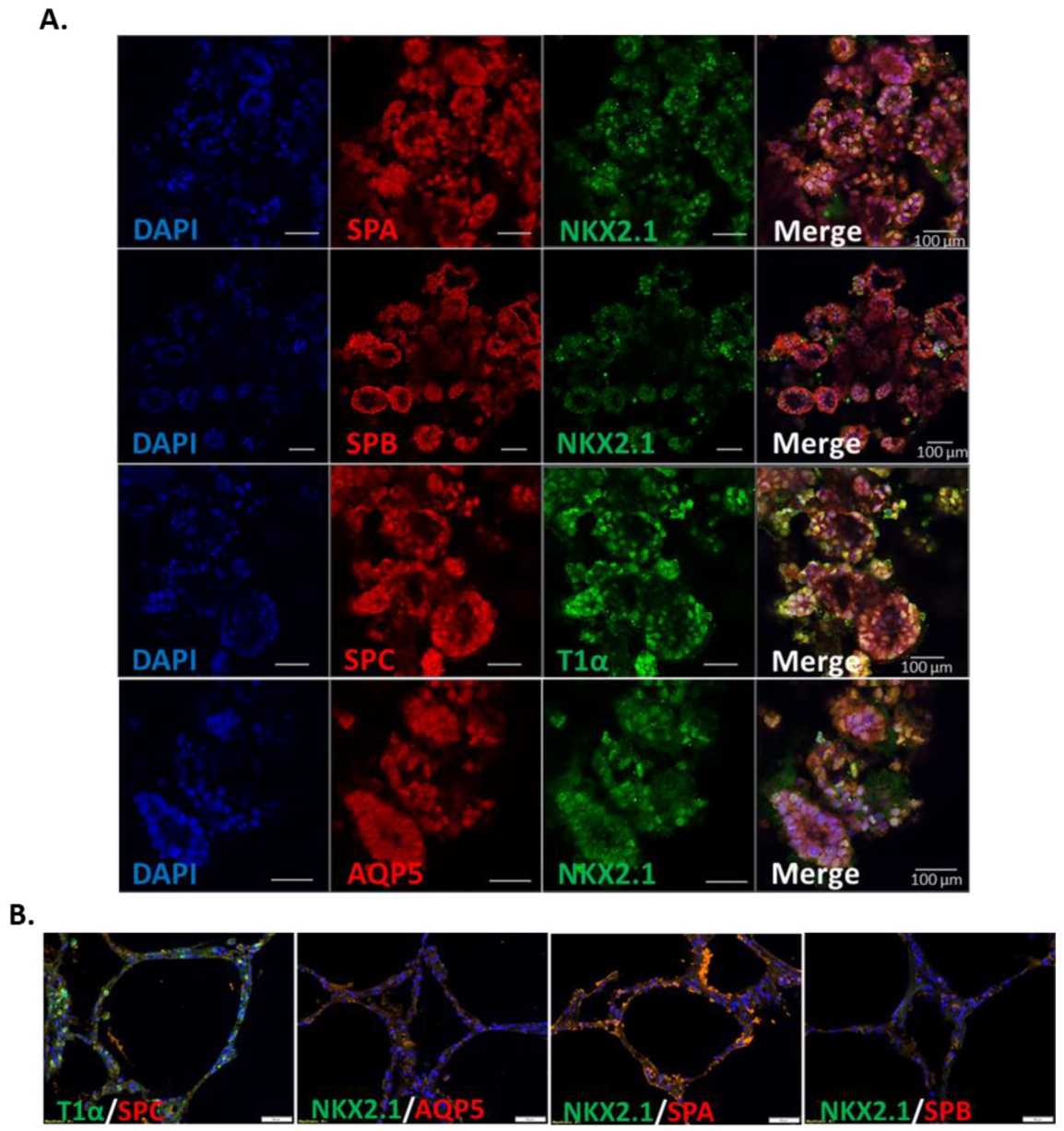 Fig. 1 Immunofluorescent staining of alveolar cell markers in iPSC-derived pulmonary organoids.1
Fig. 1 Immunofluorescent staining of alveolar cell markers in iPSC-derived pulmonary organoids.1
Reference
For Research Use Only. Not For Clinical Use.