The pregnane X receptor (PXR) and the constitutive androstane receptor (CAR) are DNA binding and ligand-regulated transcriptional factors that belong to the superfamily of nuclear receptors. Activation of either PXR or CAR initiates the expression of cytochrome P450 enzymes and other proteins involved in the metabolism and excretion of xenobiotic and endobiotic compounds. Creative Biolabs has focused on the development of computational pharmacology services for many years and has established excellent platforms for drug development. We provide a variety of nuclear receptors modeling services to meet the diverse needs of our customers.
PXR and CAR are key receptors that regulate the metabolism and detoxification of drugs and other chemicals in the human body. The CAR and PXR regulation of CYPs (phase I drug-metabolizing enzymes) is a typical mechanism of gene regulation, and these receptors are also involved in the regulation of other aspects of drug metabolism and excretion. In addition, CAR and PXR also regulate expression of phase II drug metabolism including UDP-glucuronosyl transferase (UGT), sulfotransferase (SULT) and glutathione-S-transferase (GST) enzymes. UGTs, GSTs and SULTs function to conjugate hydrophilic groups to promote the water solubility of compounds such as exogenous drug metabolites. PXR and CAR cross-talk with endogenous stimuli to regulate various aspects of liver physiology, such as cell growth, tumor development, hormone homeostasis, and energy metabolism.
The ligand-binding domain (LBD) of PXR contains 10 α-helices (H1, H3, H3’, H4, H5, H7-H10, and H12) and 5 β-strands (S1, S1’, and S2-S4). Helix H12 is also termed the activation function 2 (AF-2) helix because its conformation is the key determinant for the active status of the receptor. In the PXR structure, the AF-2 helix adopts an active position by packing tightly against the core domain of the LBD, suggesting that PXR is inclined to the activation conformation even in the absence of ligand. Ligand binding within the lower half of the LBD stabilizes the overall structure and the active conformation of the PXR, providing a mechanism for the receptor activation.
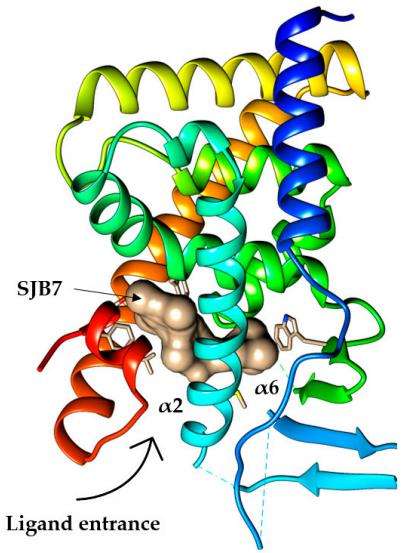 Fig.1 Human PXR crystal structures.1
Fig.1 Human PXR crystal structures.1
The LBD of CAR contains 11 α-helices, H1, H3, H3’, H4–H10, and AF-2 (which is the C-terminal activation function motif); two 310 helices (H2 and H2’) located between helices H1 and H3; and three small β-strands (S1, S2, and S3). These helices are arranged into four helical layers: three layers of α-helices that compose the core LBD and an additional layer that consists of 310 helices H2 and H2’. Importantly, the residues between helices H10 and AF-2 adopt a short helix (Hx) structure, which is tightly packed against helices H3 and H10 in the agonist-bound CAR structures. The rigid nature of helix Hx is shown to play a crucial role in maintaining the high level of constitutive activity of CAR by stabilizing the active conformation of the AF-2 helix.
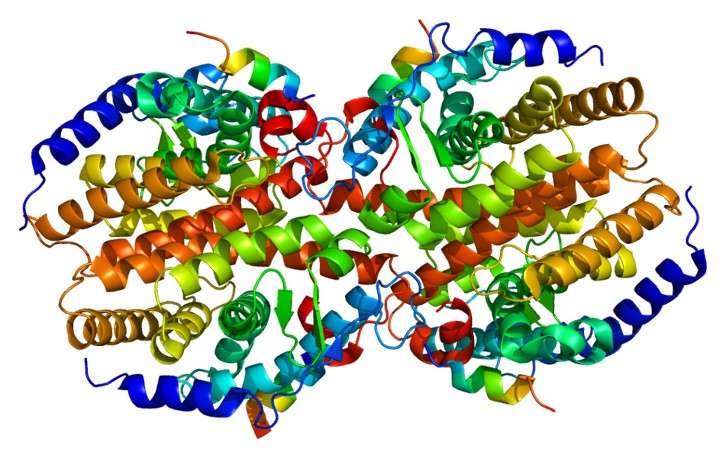 Fig.2 The CAR crystal structures.2
Fig.2 The CAR crystal structures.2
PXR is a receptor capable of binding ligands of various sizes, owing to the flexibility of its LBD. Its ligand pocket is fairly large and can expand upon the binding of large and bulky molecules. The active conformation of PXR’s AF-2 is determined partly by ligand binding and partly by coactivator binding. However, acquisition of the ligand into the proper conformation for full receptor activation is finally determined by coactivator interaction with the LBD. CAR is a constitutively active receptor due in part to its rigid LBD structure that maintains its helix-12/AF-2 in a conformation favoring interactions with its heterodimerization partner RXR. The efforts to understand the structures and functioning of CAR and PXR are revealing novel mechanisms of regulating these important nuclear receptors. This is an active area of research that will ultimately aid in our understanding of the physiological roles of CAR and PXR and for the design of therapeutic drugs that modulate these receptors to treat metabolic disorders. We can provide a variety of structure-based nuclear receptors modeling approaches to meet customers’ specific requirements.
The main aim of Creative Biolabs is to help in the advancement of our client's research and development objectives through lasting and collaborative relationships. Our team provides you with outstanding support and meets your specific needs with a professional technology platform. If you are interested in our services, please contact us for more details.
References
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
