HBV Vaccine Design Services
Creative Biolabs is a professional manufacturer of vaccines and is specialized in providing HBV Vaccine manufacturing services. Hepatitis is a general term for liver inflammation, caused by infectious or non-infectious factors. Hepatitis B virus (HBV) infection is the main cause of morbidity and mortality. Hepatitis B is an infectious liver disease that comes from hepatitis B virus infection. Acute infection may last for several weeks. If the virus stays in the body, it can cause severe chronic infections, leading to liver damage, liver cancer and death. But Hep B infection can be prevented. The current guidelines recommend high-risk populations for chronic HBV infection screening so that diagnosed individuals can carry out your proper hepatitis care. The vast majority of infected individuals without treatment, there will be risks for cirrhosis, liver failure and hepatocellular carcinoma. In uninfected individuals, HBV vaccines are safe and effective in preventing the spread of the disease. Countries with successful vaccination have been able to significantly reduce the prevalence of HBV.
Hepatitis B Virus
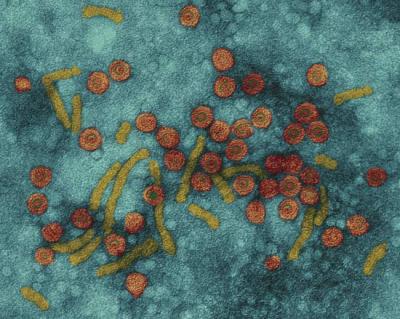
Structure
Hepatitis B virus (HBV) is a double stranded DNA virus, a member of the Hepadnaviral family, and a species of the genus Orthohepadnavirus. The virus particle is composed of an external lipid coating and an icosahedral nucleocapsid core consisting of protein. Nucleocapsid encloses viral DNA and DNA polymerase with reverse transcriptase activity similar to retrovirus. The shell contains an embedded protein that involves the binding of the virus to the susceptible cells and entry into the cells. The virus is one of the smallest envelope animal viruses with a diameter of 42 nm, but there are pleomorphic forms which lack of core, including spherical bodies and filamentous. These particles are not infectious and consist of lipids and proteins that form part of the surface of the virion, known as surface antigens (HBsAg), and are excessively produced during the life cycle of the virus.
Diseases
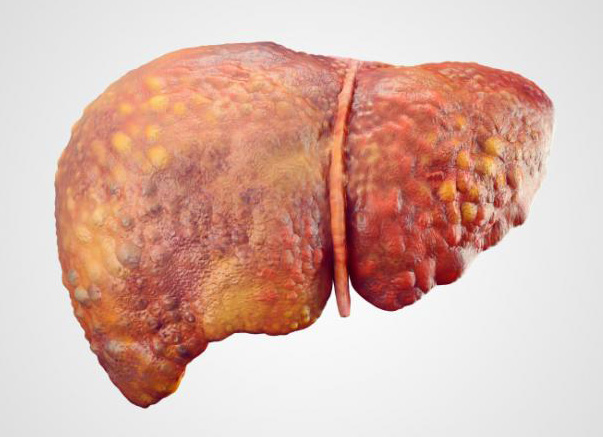
Chronic infection with HBV may lead to the disease hepatitis, cirrhosis, liver failure and hepatocellular carcinoma, and may lead to increasing risk of pancreatic cancer. Of the adults with acute HBV infection, up to 95% of them are able to recover and have no chronically infection, although they can infect others during the acute phase through the body secretions. Others become chronically infected for a long time and may infect others for much longer periods of time and have the risk of severe liver disease. Infants and children infected with hepatitis B are more likely to become long-term infections than adults and develop severe late complications.
HBV Vaccines
The FDA has licensed several HBV vaccines including several combinations vaccines. The first HBV vaccine was approved in the United States in 1981 and the recombinant version appeared on the market in 1986. It is the most effective and safe drugs required for health systems, which is in the list of essential medicines of the World Health Organization.
In 1963, the American geneticist Baruch Blumberg discovered "Australian Antigens" (now known as HBsAg) in the serum of Aboriginal people in Australia. In 1968, virologist Alfred Prince found that the protein was part of a virus that caused "serum hepatitis" (hepatitis B). Merck's microbiologist Maurice Hilleman yield a product that could be used as safe vaccines with three methods of strict filtration (pepsin, urea and formaldehyde). The vaccine was approved in 1981. Hilleman makes HBV vaccine by injecting hepatitis B surface protein into the patient. These extra superficial immune systems, which recognize surface proteins as foreign, will manufacture specially shaped antibodies that are custom-made to bind to and destroyed by these proteins. As a result, if the patient infected with HBV in the future, the immune system can produce protective antibodies promptly that destroy the virus infection to prevent further infection.
Chiron's research director, Pablo DT Valenzuela, successfully made the antigen in yeast and invented the world's first recombinant vaccine. The vaccine contains one of the viral envelope proteins, hepatitis B surface antigen (HBsAg). It was developed by inserting the HBV gene coding for the surface protein into the yeast, which only produce non-infectious surface proteins, without any risk of invading the actual viral DNA into the final product. An immune system antibody to HBsAg is established in the bloodstream. This antibody and immune system memory then provide immunity to HBV infection. This recombinant vaccine is still in use today.
All of our products can only be used for research purposes. These vaccine ingredients CANNOT be used directly on humans or animals.


