Creative Biolabs proposes contracts with guaranteed results for the hybridoma development and antibody validation for enzyme-linked immunosorbent assay (ELISA) and lateral flow immunoassay (LFIA) research use. Our function-guaranteed antibody package will satisfy your need for quality antibodies that generate positive ELISA and/or LFIA results.
ELISA is a powerful immunoassay method that occupies an irreplaceable position in the specific detection of a variety of analytes, such as peptides, proteins, antibodies, and small molecules. Currently, direct ELISA, indirect ELISA, and sandwich ELISA are several common formats. ELISA is mainly used to detect antigens recognized by antibodies or to detect antibodies that recognize antigens. In general, sample type and detection conditions are crucial factors during the validation process of ELISA antibodies. ELISA typically consists of several steps, starting with coating the ELISA plate with antigen, and followed by blocking unbound sites to avoid false positive results. Afterward, primary and enzyme-linked secondary antibodies are subsequently added to the wells to confirm positive reactions.
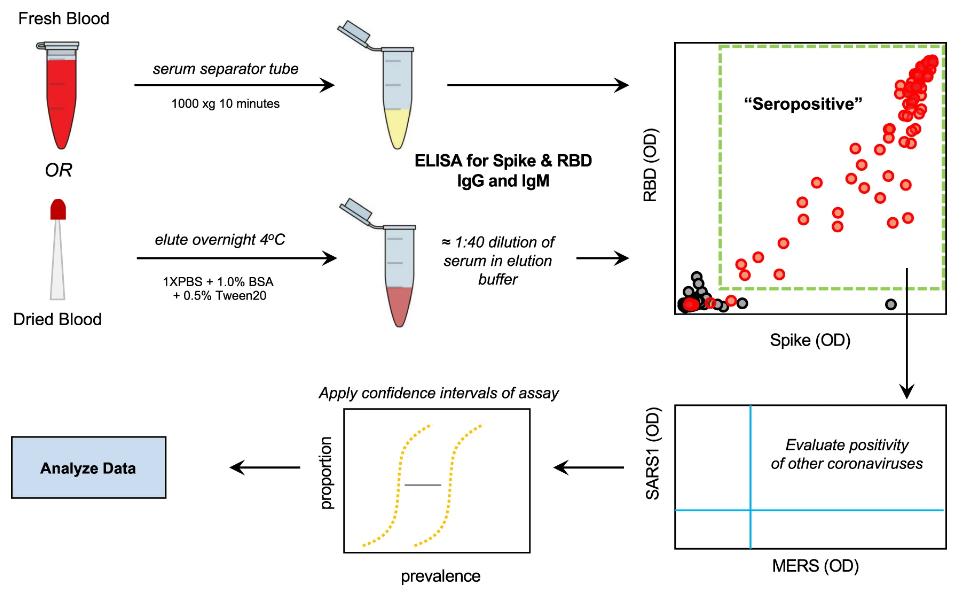 Fig.1 Serology testing protocol.1
Fig.1 Serology testing protocol.1
LFIA can be used for the detection of a variety of analytes, such as whole blood, plasma, sweat, saliva, and urine. The most prominent advantage of LFIA is that it generally does not require special instruments to obtain results. The fact that LFIA is qualitative leads to its simple determination. In addition, LFIA has received widespread attention due to its ability to achieve faster and more cost-effective detection.
The majority of hybridoma projects use indirect ELISA to screen hybridomas after HAT selection. However, ELISA-guaranteed antibody generally refers to two antibodies that can be used in a sandwich ELISA or two antibodies that bind different epitopes. Selecting a pair of capture antibodies and detection antibodies is very technical. Only pairs with high specificity, selectivity, and consistent linearity of dilution for the target will be used for further development. Plasma, serum, or tissue lysate will be used to validate antibody selectivity. In many cases, purified recombinant target proteins will be added to the biological matrix, which should be recovered and detected. The recovery observed for the spike should be almost identical in both the biological matrix and the standard diluent for a sample matrix to be considered valid for our ELISA assay. The development of LFIA functional antibodies generally involves the use of a chromatography platform to verify the function of the antibody, after hybridoma is subcloned and cryopreserved and the desired antibody is purified.
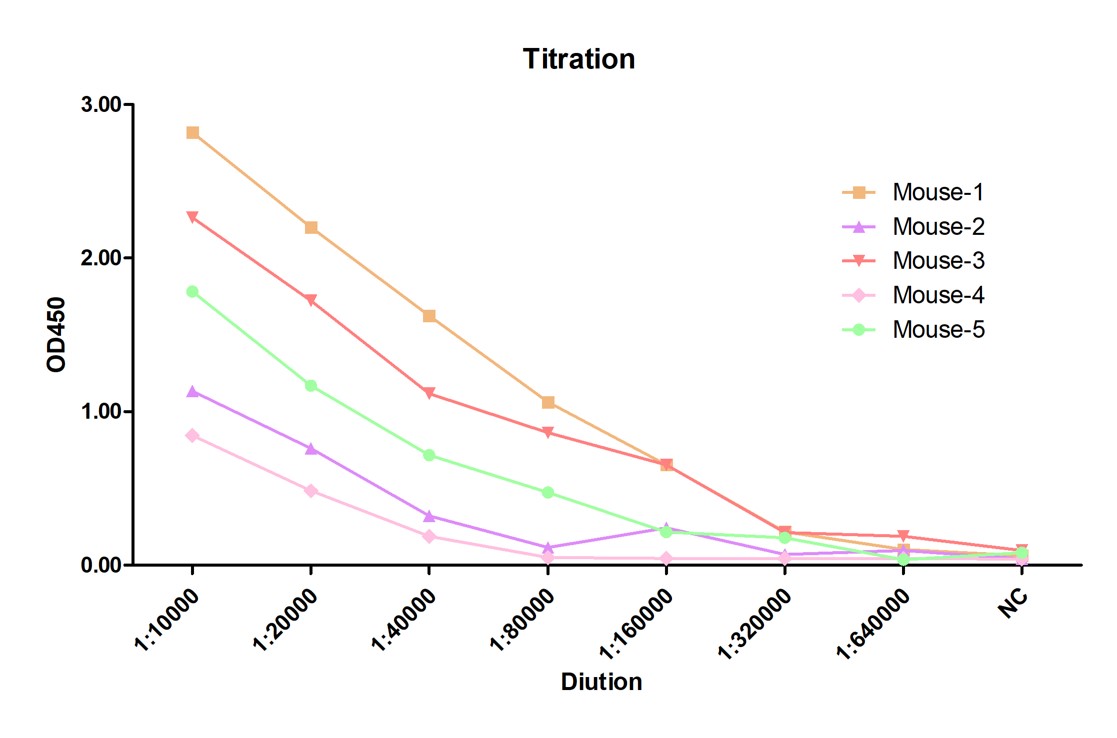
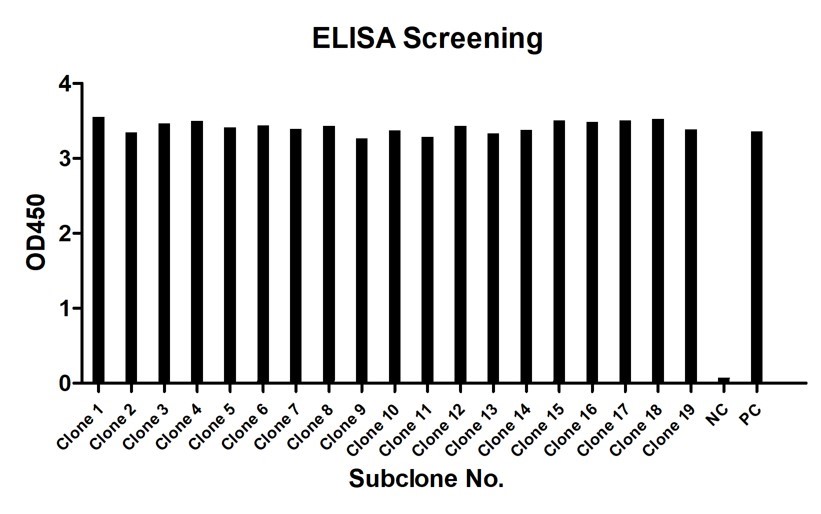
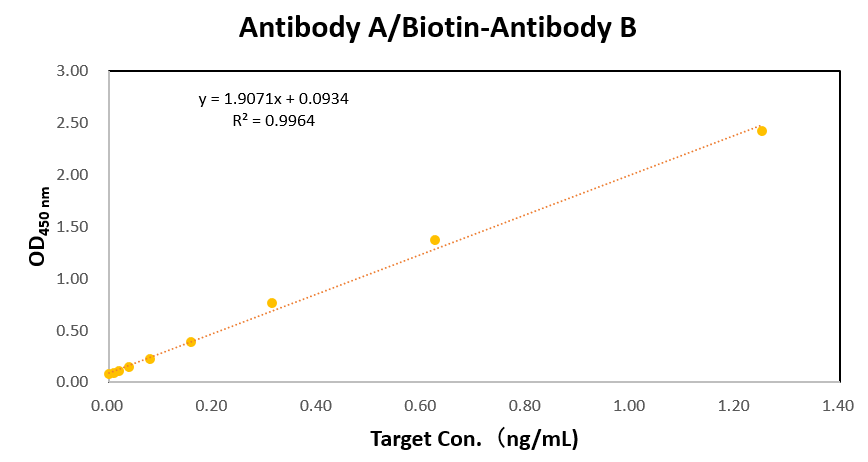
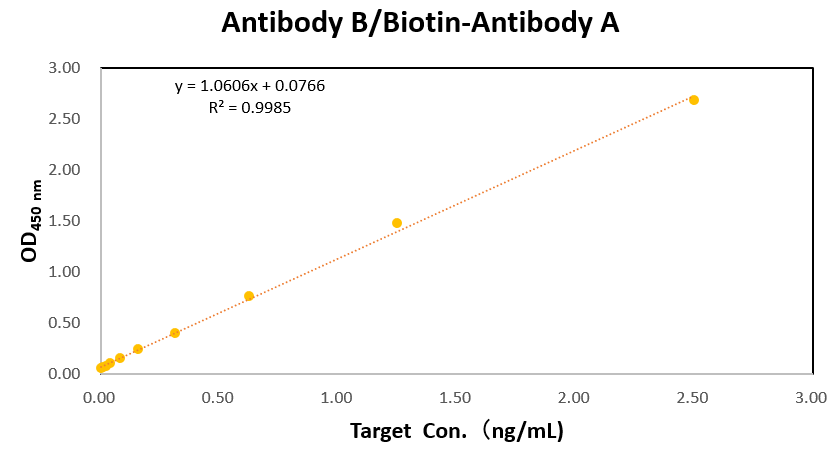
Creative Biolabs offers a diverse portfolio of antibodies with guaranteed results for ELISA and LFIA testing. Creative Biolabs also provides fully equipped manufacturing platforms for the industrial production of antibodies. If you are interested in our hybrid antibody production and functional verification services, please contact our team of antibody R&D experts for professional advice.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
