Safety Evaluation Service for Candidate Exosome Drug
Creative Biolabs is a biotechnology company focusing on biotechnology and drug development, dedicated to helping customers accelerate the development of new drugs. In recent years, exosomes have received extensive attention as a potential means of intercellular communication and signaling. Exosomes have diverse sources and compositions. Different therapeutic effects can be achieved by changing the composition and structure of exosomes. Therefore, exosomes are considered as potential new drug carriers. However, the safety evaluation of exosome drugs still faces some challenges and difficulties.
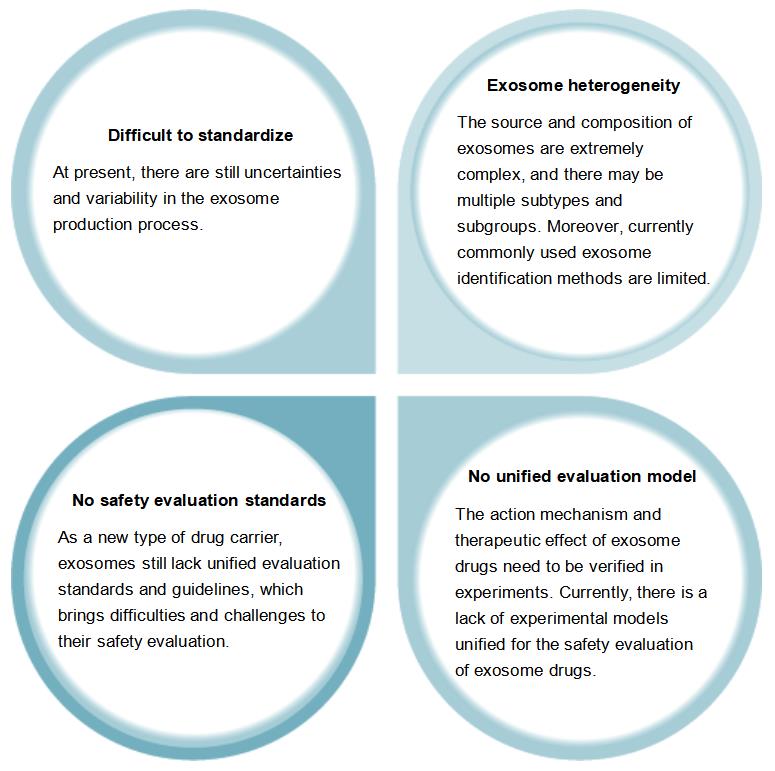
The Safety Evaluation of Candidate Exosome Drug Still Faces Some Challenges
Comprehensive Safety Evaluation Service for Candidate Exosome Drug at Creative Biolabs
The safety evaluation of exosome drugs needs to comprehensively consider their physiological activity, pharmacokinetics, immunogenicity, toxicology, and other factors. Creative Biolabs provides a complete set of safety evaluation services for candidate exosome drugs, aiming to provide customers with comprehensive, efficient, and reliable exosome drug safety evaluation solutions based on exosome drug safety evaluation solutions based on animal models and cell models.
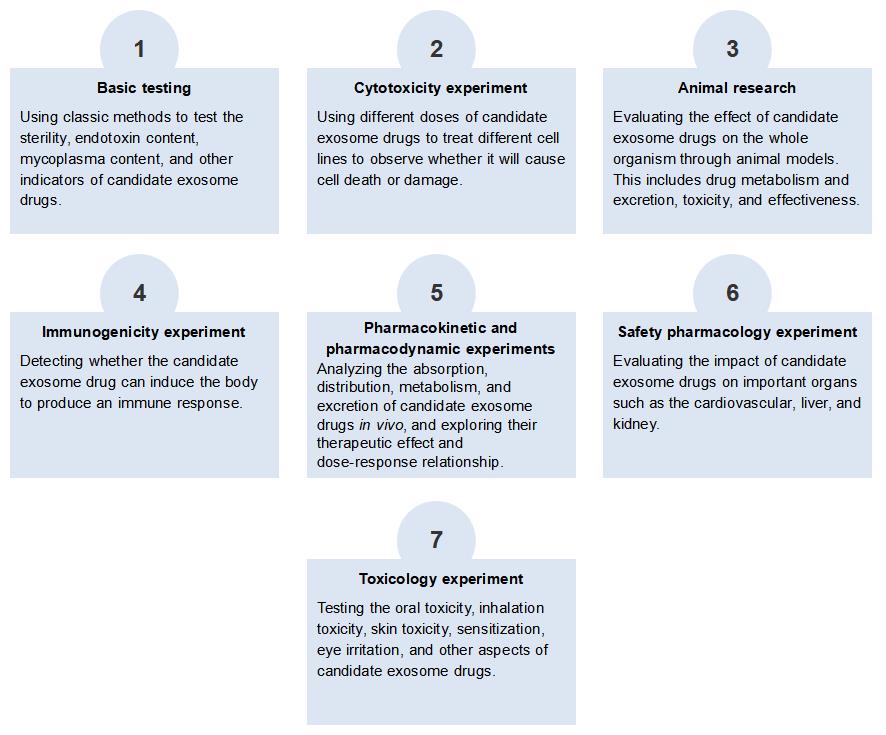
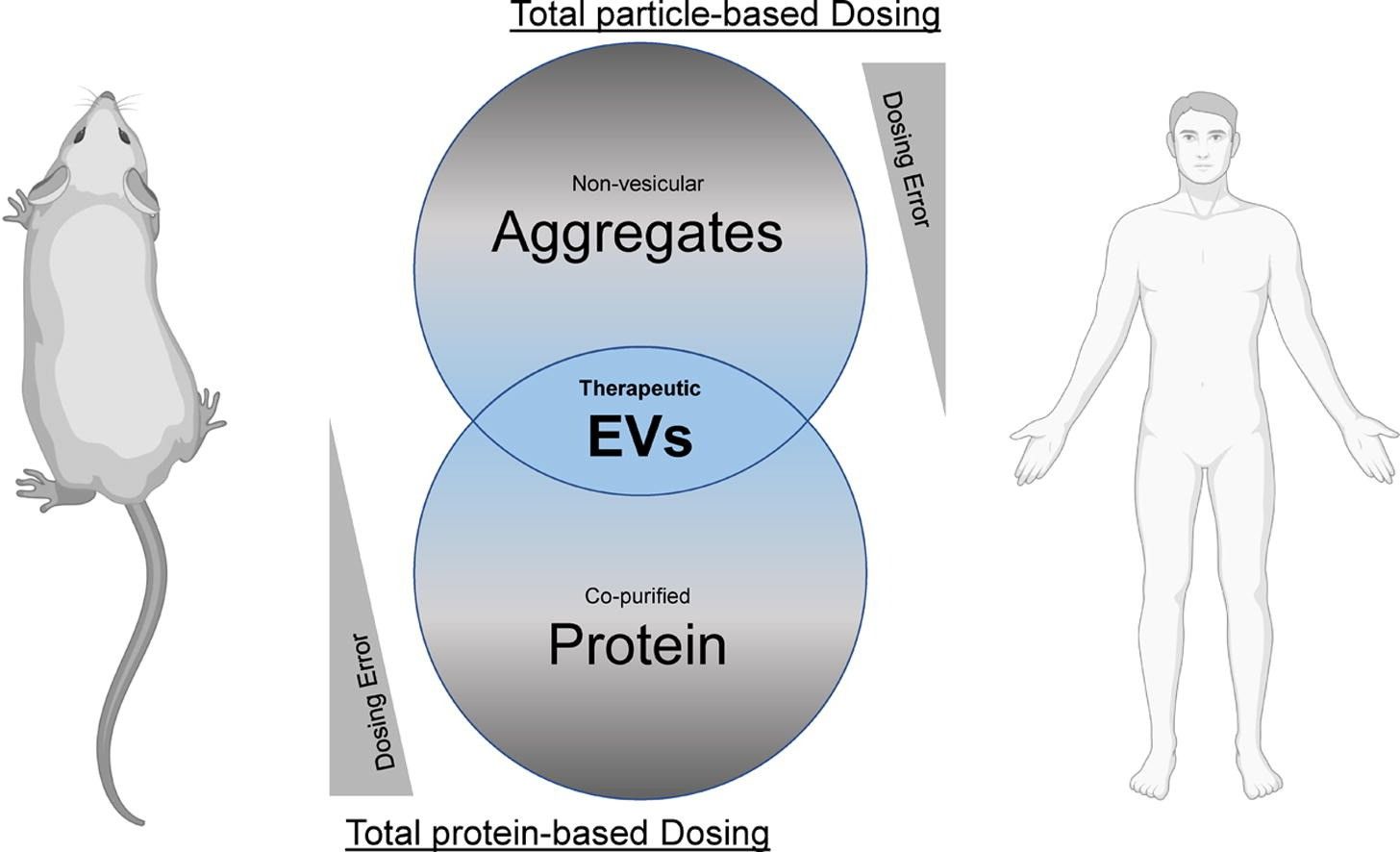 Fig.1 Dose discrepancies depending on the disease model.1,2
Fig.1 Dose discrepancies depending on the disease model.1,2
If you are looking for a reliable biotech company to evaluate the safety of your exosome drug candidates, then Creative Biolabs will be your partner of choice. Please contact us. We conduct various tests in strict accordance with the safety evaluation standards of biological products so that you can refer to the safety and effectiveness of candidate drugs in the research stage.
References
-
Yang, GH.; et al. Overcome the barriers of the skin: exosome therapy. Biomaterials Research. 2021, 25(1):22.
-
under Open Access license CC BY 4.0, without modification.
For Research Use Only. Cannot be used by patients.
Related Services:



 Fig.1 Dose discrepancies depending on the disease model.1,2
Fig.1 Dose discrepancies depending on the disease model.1,2








