Therapeutic Exosomes for Retinal Repair
Various types of retinopathy are the main cause of various high levels of visual impairment, including age-related macular degeneration (AMD), diabetic retinopathy (DR), retinitis pigmentosa (RP) and other ocular diseases, which seriously affect the quality of human life. Special sources of exosomes have been shown to mimic the optic neuron survival-promoting and optic neuroprotective effects of their parent cells, such as stem cells and olfactory ensheathing cells (OECs). Creative Biolabs can provide exosome research services related to retinal repair and aims to actively promote retinal repair-related research by researchers from around the world.
Retinopathy Diseases
AMD is characterized by an irreversible progressive loss of central vision and is divided into two types: atrophic AMD and exudative AMD. In atrophic AMD, atrophy of the macular retinal pigment epithelium (RPE) and choroidal capillaries occurs, along with vitreous formation. The production of choroidal neovascularization (CNV) triggers secondary exudation and retinal hemorrhage, causing rapid loss of central vision or even loss of vision in patients with exudative AMD in a short period of time.
The development of DR is caused by metabolic disturbances induced by abnormally high levels of blood glucose and is divided into non-proliferative DR (NPDR) and proliferative DR (PDR). The accumulation of the risk of DR is influenced by the degree of progression of diabetes mellitus, with damage to the retinal capillary endothelium inducing vascular occlusion, lipid exudation and retinal hemorrhage in the early stages of the disease, followed by the development of retinal edema, ischemia and neovascularization in the late stages. angiogenesis, which is the pathological change of progressive deterioration of NPDR to PDR. At the same time, the thickened retina and its nerve fluid accumulation induce diabetic macular edema (DME), which is another feature of DR.
In addition to AMD and DR induced by retinopathy, RP is another ocular disease with inherited retinal degeneration associated with nearly 80 gene mutations. By knocking out genes such as RP2, CNGB1, SPATA7, LRAT or deletion of non-coding RNAs such as miRNA-96/182/183 in mice, RP-related disease features, including thinning of the outer nuclear layer and diminished response to retinal electrical signals, can be mimicked.
Conventional Methods of Retinal Repair
Drugs and surgery are the traditional methods of treating retinopathy. For example, anti-vascular endothelial growth factor is used to inhibit neovascularization and promote vascular atrophy, but may induce negative events such as retinal fibrosis and scar formation in the long term. Pharmacological treatments such as antioxidant enzymes to reduce oxidative stress and calcium phenobenzenesulfonate to improve fundus microcirculation also carry the safety risks of long-term drug resistance. In addition, gene therapy is a promising therapeutic strategy. LncRNA MALAR1 may reduce microvascular damage by inflammation through epigenetic regulation of pro-inflammatory cytokines in DR. LncRNA NEAT1 has been shown to delay the progression of DR in diabetic model mice. In RP model mice, normal retinal structure and function were maintained and progressive loss of photoreceptors was improved by Bcl-2 knock-in.
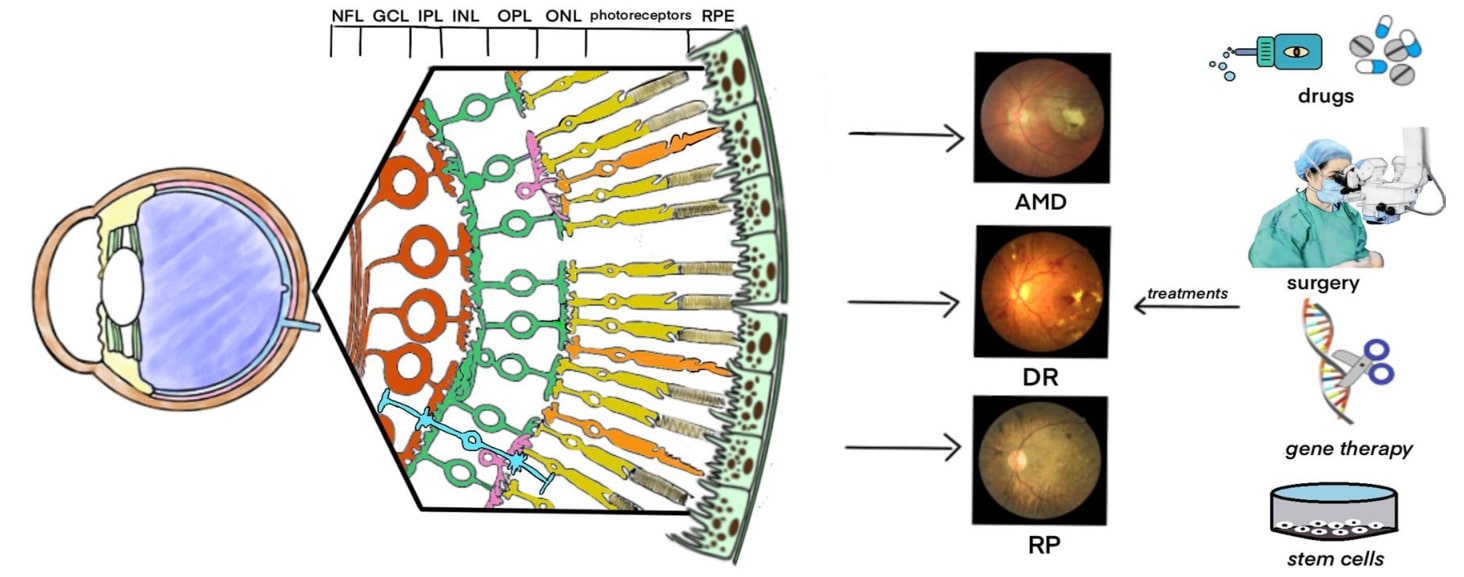 Fig.1 Retinal disorders and treatments. (Yu, 2022)
Fig.1 Retinal disorders and treatments. (Yu, 2022)
Therapeutic Exosomes for Retinal Repair
Functional analysis of embryonic stem cells (ESCs) and mesenchymal stem cells (MSCs) has shown that these stem cells have intrinsic properties to modulate immunity and anti-inflammation, promote secretion of neurotrophic factors, and increase regeneration of retinal cells, thereby accelerating cell replacement and functional recovery at the site of damage. The advanced nature of stem cell therapies has been demonstrated by several evidences. However, co-donor pathogenicity genes, tumorigenicity and immune rejection retain their safety risks.
Exosomes have inherent properties such as low toxicity, high permeability and high biocompatibility, whether under physiological or pathological conditions. Furthermore, the ability to penetrate biological barriers and targeting preferences conferred by modifications reduces the safety risk of systemic distribution after drug administration, overcoming the limitations of general drug blockage by biological barriers. Thus, compared to stem cell therapy alone, stem cell-secreted exosomes as a non-cellular therapeutic strategy has brought light to the development of new approaches for retinal repair. For example, miRNA-126 delivered by exosomes from MSCs downregulates the high-mobility group BOX1 pathway, synergizes with miRNA-222 to downregulate the retinal inflammatory response in animal models of diabetes, and alleviates retinal damage by downregulating the VEGF-A pathway. Exosomes derived from ESCs and bone marrow stem cells (BMSCs) show neuroprotective and angiogenic inhibitory effects on retinal ganglion cells. protection and inhibition of angiogenesis, thereby preventing photoreceptor apoptosis and retinal ganglion cell dysfunction, which involves the regulation of optic nerve physiological functions by exosome-loaded cargoes such as miR-100-5p, miR-144-5p, and miR-486-5p. In addition, OECs play a key role in the regenerative expression and regular renewal of neurons, and their secreted neurotrophic and immune factors significantly improve the microenvironment for optic neuron survival, while their derived exosomes deserve further attention because they can carry similar components.
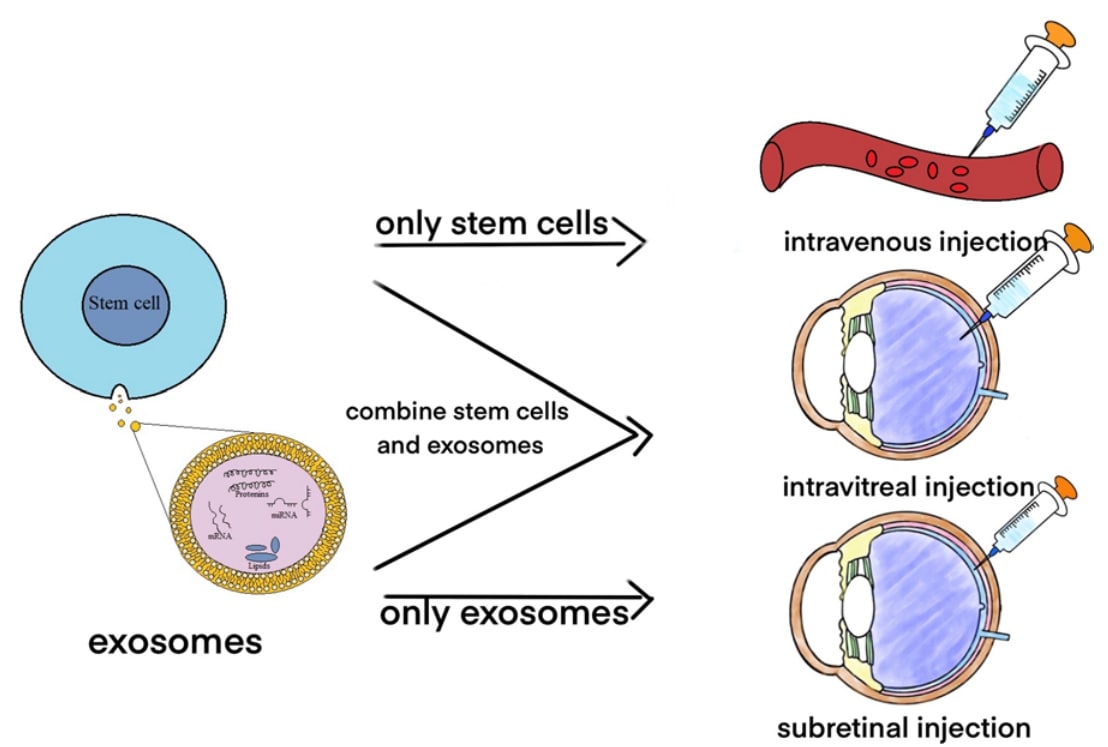 Fig.2 Use stem cells and exosomes to treat retinal disorders. (Yu, 2022)
Fig.2 Use stem cells and exosomes to treat retinal disorders. (Yu, 2022)
Compared to stem cells alone, stem cell-derived exosomes have the inherent advantages of penetrating biological barriers, low immunogenicity and non-tumorigenicity, in addition to improving the microenvironment of optic neurons and facilitating retinal repair. As a non-cellular therapeutic strategy, the application of stem cell-derived exosomes is promising. Creative Biolabs has established a variety of technical platforms related to the extraction, identification and functional analysis of stem cell-derived exosomes to provide clients with retinal repair-related exosome research services. Please contact us to advance your research.
Reference
-
Yu, Y.; et al. Update of application of olfactory ensheathing cells and stem cells/exosomes in the treatment of retinal disorders. Stem Cell Res Ther. 2022, 13(1): 11.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Retinal disorders and treatments. (Yu, 2022)
Fig.1 Retinal disorders and treatments. (Yu, 2022)
 Fig.2 Use stem cells and exosomes to treat retinal disorders. (Yu, 2022)
Fig.2 Use stem cells and exosomes to treat retinal disorders. (Yu, 2022)








