Prenatal Genetic Diagnosis
Inquiry NowPrenatal genetic diagnosis (PGD) refers to the process of detecting whether the fetus has some genetic diseases or abnormalities during fetal development by using various methods and techniques. The purpose of a prenatal genetic diagnosis is to provide prospective parents with genetic information about the fetus and to provide opportunities to adjust pregnancy management and/or postnatal care. The earliest prenatal genetic diagnosis was carried out in the 1950s, mainly by using amniocentesis to detect whether the fetus had chromosomal abnormalities or neural tube defects. With the development of science and technology, prenatal genetic diagnosis gradually appeared with more methods and techniques, such as chorionic villus sampling, fetal blood sampling, and molecular diagnostic techniques. In recent years, with the emergence of noninvasive prenatal genetic diagnosis (NIPGD), prenatal genetic diagnosis has entered a new stage.
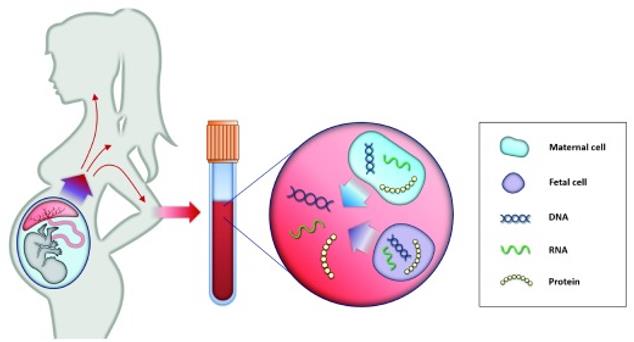 Fig.1 Principle of non-invasive prenatal testing. (Pös O, 2019)
Fig.1 Principle of non-invasive prenatal testing. (Pös O, 2019)
Amniocentesis
Amniocentesis is a method of genetic or chromosomal analysis that involves inserting a thin needle into the abdomen of a pregnant woman and extracting a small amount of amniotic fluid. Amniotic fluid is the liquid that surrounds and protects the fetus, and it contains fetal cells and other substances that can detect whether the fetus has some genetic diseases or abnormalities, such as Down's syndrome, Edwards' syndrome, Patau's syndrome, or neural tube defects.
Amniocentesis is usually performed between 16 and 20 weeks of pregnancy and is suitable for women who have a high risk of giving birth to a baby with a genetic or chromosomal disease or abnormality. These risk factors may include advanced maternal age, abnormal screening test results, a family history of genetic diseases, or a previous pregnancy with a genetic disease. Amniocentesis has high accuracy, reliability, and information content and can diagnose more than 100 different genetic or chromosomal diseases or abnormalities, as well as assess fetal lung maturity and infection status. However, amniocentesis also has disadvantages and risks, such as being invasive, painful, and costly, and may cause miscarriage (about 0.5% to 1%), bleeding, infection, maternal-fetal injury, or amniotic fluid leakage. Therefore, pregnant women should fully understand the pros and cons of amniocentesis before undergoing it and make decisions based on their own situation and preferences.
Chorionic Villus Sampling
Chorionic villus sampling (CVS) is a method of genetic or chromosomal analysis that involves inserting a thin needle or catheter into the abdomen of a pregnant woman and taking a small piece of tissue from the placenta. The placenta is the organ that connects the mother and the fetus and provides nutrients and oxygen to the fetus. The placental tissue contains chorionic villi, which are tiny finger-like projections that have the same genetic makeup as the fetus.
CVS is usually done between 10 and 13 weeks of pregnancy and is indicated for women who have a high risk of having a baby with a genetic or chromosomal condition, such as Down's syndrome, Edwards' syndrome, Patau's syndrome, or other inherited disorders. The risk factors may include advanced maternal age, abnormal screening test results, a family history of genetic disorders, or a previous pregnancy with a genetic condition. CVS has the advantages of being done early in pregnancy, providing fast and accurate results, and being able to detect more than 200 different genetic or chromosomal conditions. However, CVS also has some disadvantages and risks, such as being invasive, causing discomfort and cramping, and requiring a follow-up blood test to screen for neural tube defects. It also carries a small risk of pregnancy loss (about 0.5% to 1%), bleeding, infection, injury to the fetus or the mother, or limb defects in the baby (especially if done before 10 weeks). Therefore, pregnant women should fully understand the pros and cons of CVS before undergoing it and make decisions based on their own situation and preferences.
Fetal Blood Sampling
Fetal blood sampling (FBS) is a method of taking a small amount of blood from the fetus during pregnancy by accessing the fetal blood vessels in the umbilical cord, liver, or heart. FBS can be used for genetic or chromosomal analysis, as well as for assessing or treating fetal blood status.
FBS is usually done between 18 and 34 weeks of pregnancy and is indicated for cases where other prenatal tests are not possible or not conclusive. FBS can diagnose or treat various genetic or chromosomal conditions, such as Down's syndrome, Edwards' syndrome, Patau's syndrome, or other blood problems. However, FBS also has some disadvantages and risks, such as being invasive, painful, and expensive. It also carries a small risk of pregnancy loss (about 1% to 2%), bleeding, infection, injury to the fetus or the mother, or fetal distress.
Prenatal Diagnosis of Genetic Disorders
Prenatal diagnosis of genetic disorders is the process of detecting whether the fetus has inherited a specific gene mutation or chromosomal abnormality that may cause a disease or a malformation. The purpose of prenatal diagnosis of genetic disorders is to provide prospective parents with accurate and timely information about the genetic status of their fetus and to offer them options for pregnancy management and/or postnatal care.
Prenatal diagnosis of genetic disorders can be performed by using various methods and techniques, depending on the type and timing of the disorder, the availability and accuracy of the tests, and the preferences and values of the parents. The main methods and techniques include preimplantation genetic testing (PGT), cell-free fetal DNA testing (cffDNA), chorionic villus sampling (CVS), amniocentesis, and fetal blood sampling (FBS). These methods and techniques have different advantages, disadvantages, and risks, as well as different diagnostic capabilities and applications.
Reference
- Pös O, et al. Recent trends in prenatal genetic screening and testing. F1000Res. 2019;8:F1000 Faculty Rev-764.
