Dedicated to assisting our clients in obtaining optimized hybridoma cell lines, Creative Biolabs provides hybridoma cell line optimization services that aim to increase and stabilize the productivity of your original cell line for monoclonal antibody (mAb) production. Regarding your expectations, our innovative technology will be applied to your specific clone or pool, to maintain the high quality of your antibody products.
Hybridoma technology is a method that allows large-scale production of mAbs to selective antigens, via splenocyte cell fusion from immortalized myeloma-derived cell lines in immunized animals. The obtained hybridoma cells can be screened, and single cells cloned to yield an immortal hybridoma cell line for the following long-term production of specific mAbs. During the past decades, hybridoma technology has been developed benefiting from the advancement of modern cell culture practices, including optimized growth supplements and cell medium formulations, achieving more consistent mAb production.
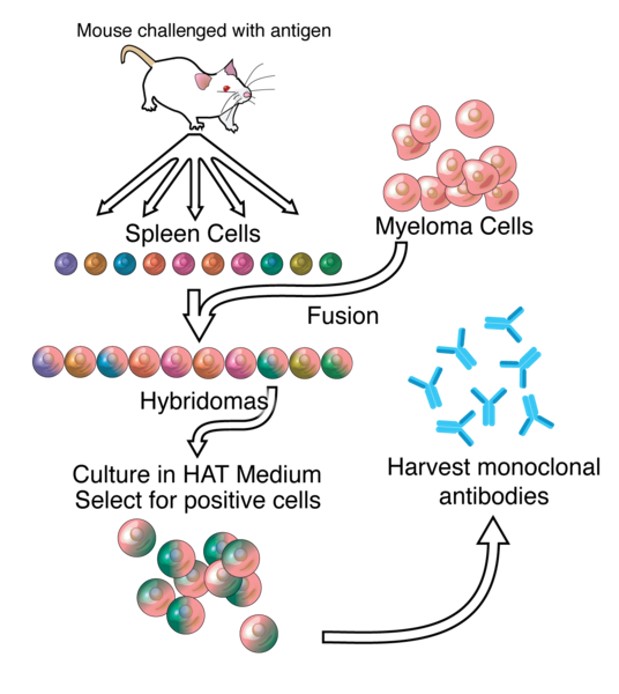 Fig.1 Overview of hybridoma technology and monoclonal antibody creation. Distributed under Open Access license CC BY-SA 3.0, from Wiki, without modification.
Fig.1 Overview of hybridoma technology and monoclonal antibody creation. Distributed under Open Access license CC BY-SA 3.0, from Wiki, without modification.
Although hybridoma technology has been evolving persistently, there remains a need for cost-effective, animal-derived culture supplements to support early hybridoma cell survival, cloning, and growth. Cell metabolism and respiratory parameters can be individually and subtly adjusted to improve the expression level and quality of the interested protein. Besides, mutations happen over time in the population of cell lines, bringing possibilities to the occurrence of virulent, non-antibody-producing cells and threatening the security of the cell line. The condition is defined as cell line drift and highlights the importance of continuous monitoring and cell line certification.
At Creative Biolabs, we offer reliable and cost-effective services of hybridoma cell line optimization and prevention of cell line drift to assist in your projects. We develop customized optimizing strategies to generate the best hybridoma possible for each antibody produced. Meanwhile, large seed stocks can be maintained here for each hybridoma cell line where they are closely monitored for hybridoma viability, the absence of microbes, antibody expression, and isotype uniformity.
In order to provide maximum mAb yield during production, we will examine the viability and the clonality of the provided hybridoma through our featured methods.
Each cell line will be evaluated in the following four steps:
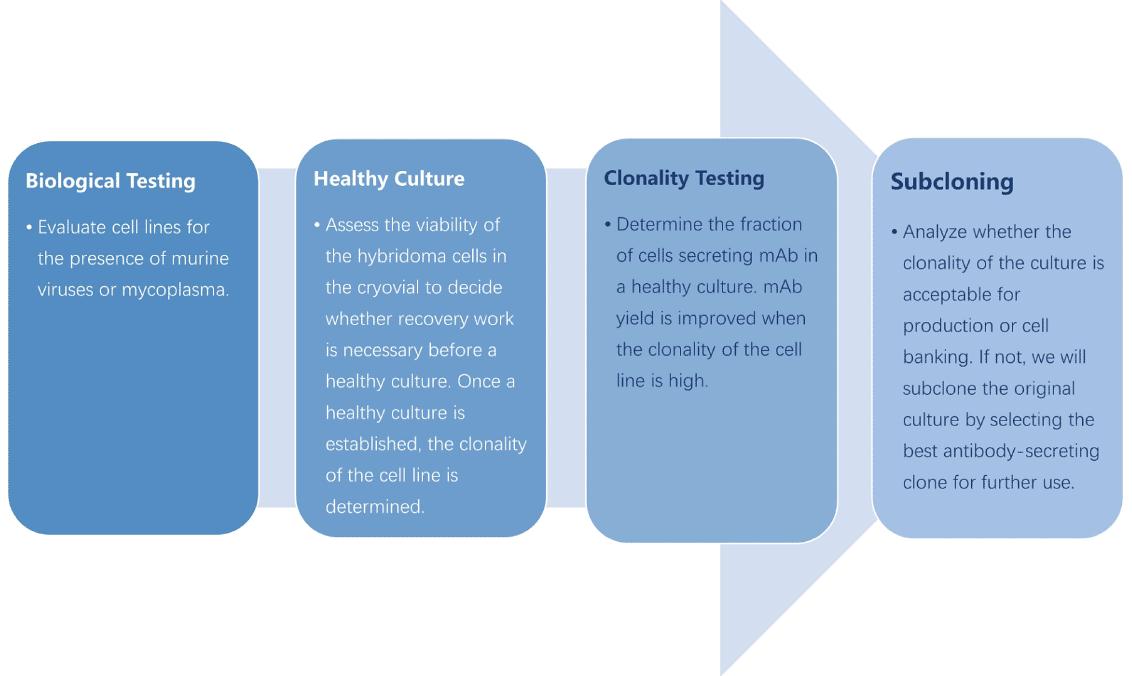
After reviving the cell line, percent viability is determined with a trypan blue contrast assay. The cell line is then subcloned in order to assess monoclonality. Antibody activity is assessed with an immunoassay and the percent of specific antibody expression is calculated. The cell line is subcloned with the limiting dilution method to reinstate monoclonality preceding the development of a new cell bank.
All cell lines are screened for mold, fungi microbial growth, mycoplasma, and viruses prior to the development of a certified hybridoma bank. Aliquots are stored to ensure the security of the cell line.
 Fig.2 Assay of PCSK9-Ab secreted from 6A6 hybridoma cells.1
Fig.2 Assay of PCSK9-Ab secreted from 6A6 hybridoma cells.1
The hybridoma technique traditionally utilizes the ELISA method to detect antibodies secreted into the supernatant of specific hybridoma cells after a culture period of 1-2 weeks. During this time, the cells undergo clonal expansion and antibody secretion to attain concentrations detectable by ELISA, a process that significantly hinders the efficiency of the technique. However, the proposed CLA, enhanced with P-RCAES, offers a superior alternative to ELISA due to its high sensitivity and specificity. In this study, Ao, Hang, et al. evaluated the performance of the CLA in detecting PCSK9-Ab secreted by 6A6 hybridoma cells.1 A single cell was isolated using limiting dilution on a 96-well plate, and supernatants from 6A6 hybridoma cells cultured for various durations were tested with both the CLA and ELISA. Notably, ELISA could not detect PCSK9-Ab from 6A6 hybridoma cells until six days post-culture, whereas the CLA successfully detected PCSK9-Ab from a single 6A6 hybridoma cell after just one day of culture. This provides a rapid method for screening specific hybridoma cells.
A: Our services include but are not limited to:
A: Our proprietary know-how is based on a succession of physical stimulations leading to improved cell metabolism and respiratory parameters required for achieving high and sustainable expression of specific mAbs.
Besides, Creative Biolabs is capable of providing optimized mouse myeloma cell lines (NS0, SP2/0, etc.) for cell fusion during the process of mAb preparation. Since each hybridoma development project is unique, successful projects will often require that immunization and screening protocols be tailored to the unique needs of the client. If you are interested in our hybridoma cell line optimization services, please feel free to contact us for more information.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
