Mesenchymal stem/stromal cells (MSCs) are promising candidates for use in cell-based therapies and therefore have attracted significant interest. However, the development of their clinical utility relies on reliable sources of MSCs, defined isolation and expansion protocols. To facilitate the research in this field, Creative Biolabs provides high-quality isolation and expansion services of MSCs that are derived from a wide range of tissues and sources.
MSCs can be derived from different sources and can be isolated from various tissues, such as umbilical cord, bone marrow, cartilage, muscle, and adipose tissues. Besides, fetal dermis tissues, various dental tissues, pancreatic tissue, breast milk, menstrual blood, and endometrium have been reported to contain MSCs as well. Additionally, the MSC pool of a tissue may be expanded by dedifferentiation of differentiated cells under certain conditions. Cells from different tissues differ in properties including cell surface markers expressed and differentiation abilities. Even populations derived from a single source may be heterogeneous and have different developmental potentials.
Creative Biolabs employs different protocols for MSC isolation.
MSCs represent a rare population in bone marrow and other tissues and thus ex-vivo expansion and manipulation are required to reach a sufficiently large scale. The most important factor of successful expansion of MSCs is the culture conditions. Creative Biolabs uses optimized and standardized complete mediums for the reproducible and reliable generation and expansion of MSCs derived from different sources. The yield and the quality of the expanded cells are both ensured.
Our MSC isolation and expansion services are aimed at providing researchers with high-quality MSCs for their research needs. Our process ensures the isolation and expansion of MSCs in a controlled and standardized manner.
Our services specifically include the following categories.
Among the available sources, BM is the most commonly used one. MSCs from bone marrow are the main cell source for tissue repair and engineering, and vehicles of cell-based gene therapy. Creative Biolabs has established standardized, reliable, and easy-to-perform protocols for isolation and culture of BM-MSCs. Using our protocol, the isolated BM-MSCs exhibit multiple lineage differentiation potentials.
Blood has become a very attractive potential source of stem cells to be studied, based on their differentiation capacity and therapeutic potential. Creative Biolabs offers MSCs isolation from peripheral blood, umbilical cord blood, and menstrual blood.
The placent and umbilical tissues can be easily attained as promising sources of stem cells. These populations of MSCs have advantages such as greater proliferative capacity and immunosuppressive properties than maternal MSC populations.
Because of their easy isolation and relative abundance, adipose tissues have become a reliable source of MSCs. These cells have multiple mesodermal lineage differentiation potentials in vitro, evidenced by adipogenic, osteogenic, and chondrogenic phenotype display when cultured in the presence of established lineage-specific differentiation factors. Besides, they can also differentiate into nervous tissues, hepatic lineage, and cardiomyocytes.
MSCs can also be isolated from other tissues, such as muscles, cartilage, dental pulp, and dermis tissues. We can customize the culturing conditions and protocols to maximally expand the MSCs derived from sources while maintaining the multipotency.
Overall, our services are designed to provide researchers with reliable and consistent MSCs for their research studies. Our commitment to quality control and standardized procedures ensures that researchers can trust the integrity of the MSCs they receive from us.
Creative Biolabs employs different protocols for MSC isolation.
Creative Biolabs stands behind the high quality of our services and products. We can precisely cater to your specific requirements with our flexible, custom MSC isolation and expansion services. If you have any questions, please contact us.
Below are the findings presented in the article related to the isolation and expansion of MSC.
Isolation and expansion of human umbilical cord mesenchymal stromal cells (hUC-MSC) is a multistep process. After birth, UC is collected and stored under conditions that preserve the quality of the umbilical cord for subsequent MSC isolation. After transfer to a cell culture facility, the UC is examined and prepared for culture. The UC tissue is then cultured to allow migration of MSC from the UC debris. Upon completion of subsequent MSC expansion, the cells are cryopreserved for downstream applications, including full MSC characterization. Pia Todtenhaupt, et al. reported a reliable and standardized method for the isolation and expansion of hUC-MSC by comparing, selecting, and optimizing variables in each step of the process.
Culture media and supplements may affect the growth performance and characterization of MSC. They experimentally compared the two most commonly used combinations of media supplements. HUC-MSC showed significant differences when isolated and amplified in αMEM-hPL or DMEM-FBS media.
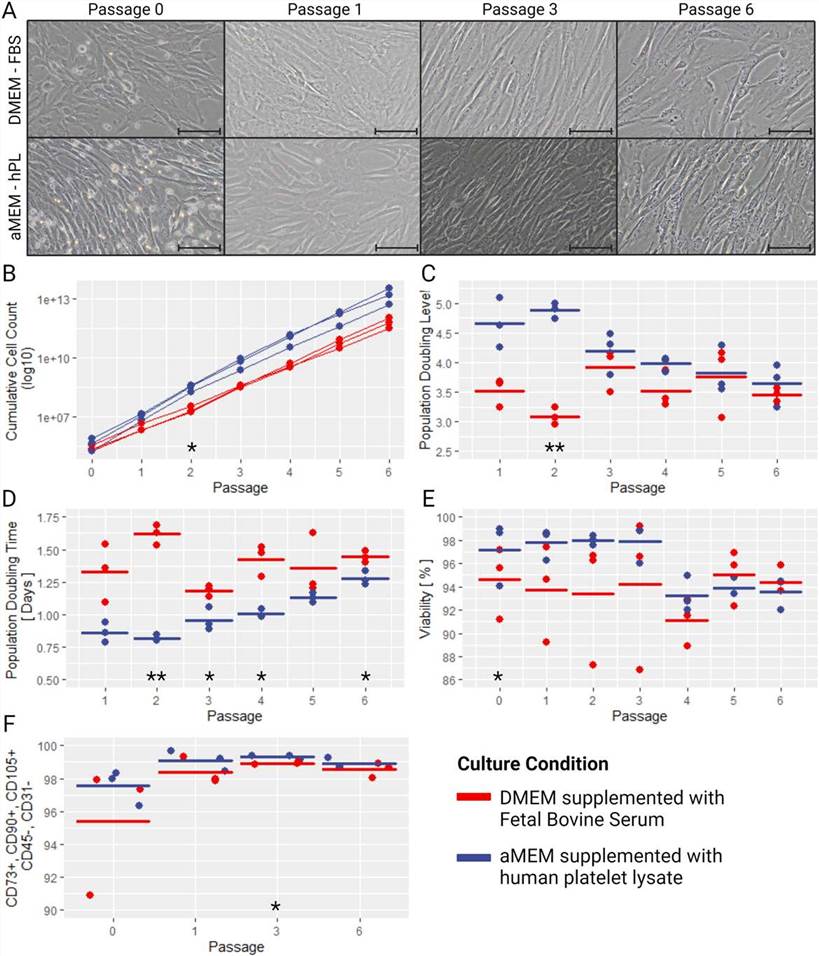 Fig. 1 The effect of serum and culture medium choice on MSC characteristics and performance.1
Fig. 1 The effect of serum and culture medium choice on MSC characteristics and performance.1
Reference
For Research Use Only. Not For Clinical Use.