GM-CSF-Secreting Tumor Cell Vaccine Development Service
Cytokines have been used to treat advanced cancers, including renal cell carcinoma and melanoma with some success, but systemic administration is associated with marked toxicity. Recombinant cytokines expressed by modified tumor cells can be used to target the cytokine to tumor or vaccination sites where they can induce a proinflammatory environment, with the additional benefit of lowering the levels of circulating cytokine and therefore circumventing the toxicity related to high systemic doses. Creative Biolabs is a world leader in the field of cancer vaccine development. We developing a range of cytokines, of which GM-CSF has been shown to have potential in allogeneic tumor cell vaccination models. With our extensive experience and advanced platform, we are therefore confident in offering the best services and the finest results for our customers all over the world.
Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF)
Granulocyte-macrophage colony-stimulating factor (GM-CSF), also known as colony-stimulating factor 2 (CSF2), is a monomeric glycoprotein secreted by macrophages, T cells, mast cells, natural killer cells, endothelial cells and fibroblasts that functions as a cytokine.
Although GM-CSF has highly pleiotropic effects, it is involved principally in stimulation of proliferation and activation of granulocytes and monocytes. GM-CSF stimulates dendritic cell maturation and is able to augment APC function. Protective immunity is induced by vaccination with allogeneic GM-CSF-secreting cells, and stimulation of cytotoxic responses to tumor cells has been demonstrated.
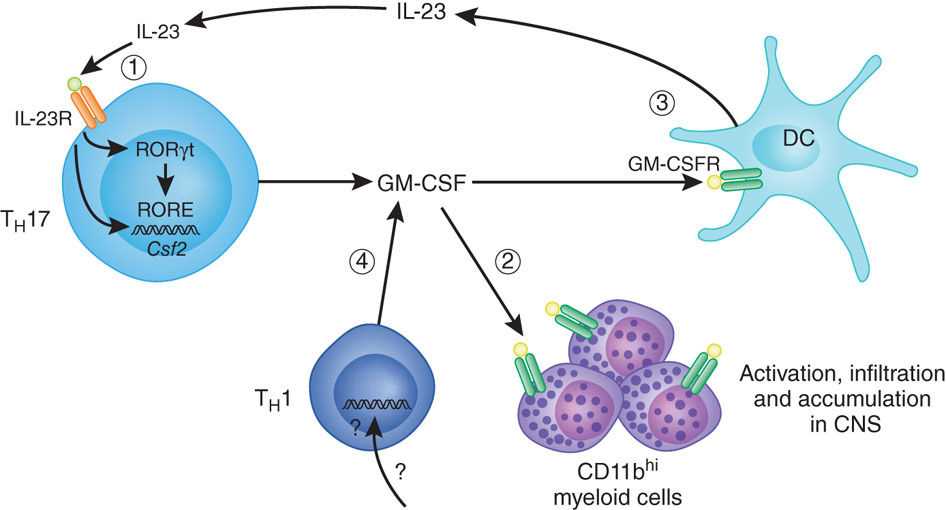
Genetically Modified GM-CSF-Secreting Tumor Cell Vaccines
Genetically modified granulocyte-macrophage colony-stimulating factor (GMCSF)-secreting tumor vaccines represent an innovative cell-based cancer vaccine platform capable of activating immunity specific for solid tumors historically considered to be inherently nonimmunogenic. An early systematic analysis compared the ability of a variety of cytokines (including IL1, IL2, IL4, IL5, IL6, GM-CSF, IFNγ, and TNFα) delivered to poorly immunogenic B16F10 melanoma cells by retroviral transduction to effect tumor rejection. GM-CSF clearly stood out as the most potent inducer of an antitumor immune response capable of mediating tumor rejection in animals with small burdens of pre-established tumors.
GM-CSF secretion at the vaccine inoculation site recruits and activates dendritic cells to take up and process antigen delivered by the tumor vaccine cells, crosspriming an effective antigen-specific immune response dependent on CD4+ T helper type 1 and 2 cells as well as CD8+ cytotoxic T cells.
Promising results in preclinical studies prompted the clinical testing of both autologous and allogeneic GM-CSF-secreting tumor vaccines as a single therapeutic intervention in patients with metastatic renal cell carcinoma, metastatic melanoma, metastatic prostate carcinoma, and early or advanced non-small cell carcinoma of the lung. These initial trials established the safety of the vaccination strategy and provided clinical validation of the relevance of antigen dose, level of GM-CSF secretion, and the cellular infiltrates at the vaccination site. The most recent clinical trials of GM-CSF-secreting cancer vaccines have tested treatment regimens that integrate these tumor vaccines into standard treatment modalities. The first reported clinical trial of GM-CSF-secreting tumor vaccines integrated with combined modality therapy for the treatment of pancreatic cancer was conducted by Jaffee and colleagues. Clinical trial of GM-CSF-secreting tumor vaccine integrated with combined modality therapy for the treatment of pancreatic cancer has been reported.
Creative Biolabs is a leader in the field of vaccine development and has focused on the cancer vaccines for years. We have experienced experts and advanced platforms that are able to provide excellent services. If you are interested in our services, please contact us for more details.
All of our products can only be used for research purposes. These vaccine ingredients CANNOT be used directly on humans or animals.


