Creative Biolabs has been dedicated to leveraging extensive efforts in the development of virus-like particles (VLPs)-based vaccines. During the past decades, we have established multiple leading-edge VLPs technology platforms. Focused on VLPs application with an expanding spectrum, we can guarantee our clients with the most efficient and reliable service of VLP-based vaccines development.
Virus-like particles (VLPs) are multimeric nanostructures assembled from native viral structural proteins but are devoid of any genetic material. VLPs are composed of high-density displays of viral surface proteins and as such VLPs are a highly adaptable tool for various applications (e.g. antibody development, delivery system, membrane protein expression).
Traditional vaccines have been prepared from inactivated or attenuated viral strains while VLPs which lack the viral genome, can provide a much safer alternative. Furthermore, they offer a polyvalent structure that can accommodate multiple copies of antigens and are able to elicit more potent immune responses. VLPs can also ensure tissue-specific targeting since they are originated from the native virus. These stable and versatile nanoparticles show excellent adjuvant properties capable of inducing innate and cognate immune responses. They present both high-density B-cell epitopes for antibody production and intracellular T-cell epitopes for inducing potent humoral and cellular immune responses. Currently, many VLP-derived vaccines have been licensed and commercialized in the market, and some others entered clinical trials.
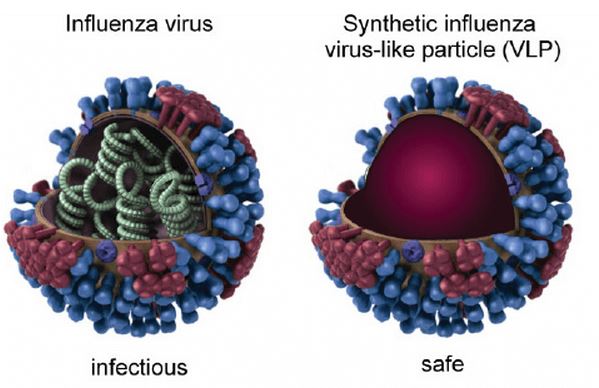 Figure 1. The influenza virus structure (left) and synthetic influenza VLPs (right). (Bioessays, 2011)
Figure 1. The influenza virus structure (left) and synthetic influenza VLPs (right). (Bioessays, 2011)
Creative Biolabs has used the most commonly applied adjuvant aluminum salts to enhance the antigen immunogenicity and excipients for various purposes such as reduction of VLPs aggregation and preservative. In order to solve the problem that samples need to be frequently removed in fermentation followed by the processes such as cell centrifugation, cell lysis, and purification, we have developed a high-throughput platform which can perform VLPs production with high yield and purity in laboratory/manufactural scale. Our world-class VLP vaccine platform has been optimized to guarantee:
Creative Biolabs now offers high-quality VLPs-based vaccines development service for worldwide customers. Our seasoned scientific staff will work closely with you to keep you apprised of project progress while taking all your goals into account. Moreover, a diverse family of in-house VLPs products is also available on our website (e.g. HBV VLP, HIV VLP, HPV VLP, CA16 VLP, EV71 VLP, poliovirus VLP, polyomaviruses VLP, AAV VLP, bacteriophage Qβ VLP, Zika VLP, etc.). We warmly welcome customers to consult and discuss their projects. Please feel free to inquire us for further discussions.
References
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
