Complement C9 C9 Function C9 Deficiency C9 Therapeutics Resources
The complement system plays a critical role in innate immunity, acting as a first line of defense against pathogens. One of its terminal components, complement C9, is essential for the formation of the membrane attack complex (MAC), which is a pivotal element in the lytic pathway of the complement cascade.
What is Complement C9?
Complement C9, also referred to as complement component 9, is the terminal protein in the complement cascade. It is a 71-kDa glycoprotein primarily synthesized in the liver and secreted into the bloodstream. Complement C9 operates synergistically with other complement proteins to form the MAC, which targets and eliminates microbial pathogens by creating transmembrane channels that disrupt their cellular integrity.
Structure of C9 Component
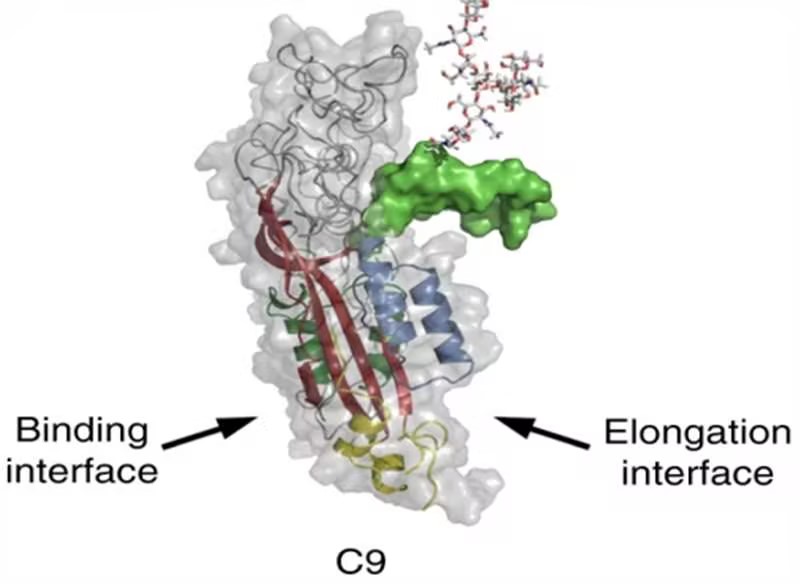 Fig.1 Structure of complement component 9.1,3
Fig.1 Structure of complement component 9.1,3
Complement C9 belongs to the membrane attack complex/perforin (MACPF) protein superfamily. Structurally, C9 is a multidomain protein containing an N-terminal type 1 TSP domain, an LDL receptor class A repeat, many potential transmembrane regions, and a C-terminal EGF-like domain. Hydrophilic analysis of the sequence showed that the N-terminal half of C9 was mainly hydrophilic, while the C-terminal part was hydrophobic. The amphiphilic structure of the primary structure is consistent with the known potential of polymeric C9 to penetrate lipid bilayers, resulting in the formation of transmembrane channels.
-
MACPF domain: This domain is responsible for membrane insertion and pore formation. Upon activation, the MACPF domain undergoes conformational changes, enabling its assembly into a transmembrane pore.
-
C-terminal domains: These regions are crucial for stabilizing the polymerized C9 complex and ensuring proper pore architecture.
When multiple C9 molecules polymerize, they form a ring-like structure with a central lumen capable of penetrating lipid bilayers. This pore structure is the hallmark of the lytic action of the MAC.
Complement C9 in the Complement Cascade
The complement system comprises over 30 proteins, including complement C1 to C9, which are activated sequentially in a cascade. This cascade can be initiated via three distinct pathways: the classical, alternative, and lectin pathways.
C9 is one of the components of the MAC, which completes a series of events leading to the destruction of the target membrane. MAC is assembled by sequentially binding complement components C5b, C6, C7, C8 and a variable number of C9 molecules to the target cell membrane. Up to 18 C9 molecules can bind to each C5b-8 complex, forming stable ion pores or channels on the membrane and causing lysis and death of the target cell upon complement activation.

Fig. 2 Assembly of the membrane attack complex (MAC).2, 3
What is the C9 Complement Gene?
The C9 gene is located on chromosome 5p13 in humans and spans approximately 15 kilobases. It comprises several exons encoding the 559 amino acid residues of the mature C9 protein. Therefore, mutations or polymorphisms in the C9 complement gene can have profound implications for immune function.
-
Frameshift mutations, nonsense mutations, or deletions within the C9 gene result in C9 deficiency (a rare autosomal recessive disorder characterized by a lack of or reduced activity of C9).
-
Certain single nucleotide polymorphisms (SNPs) in the C9 gene have been associated with age-related macular degeneration (AMD) and variability in response to infectious agents.
What is the Function of Complement Component 9?
Structurally, C9 belongs to the cholesterol-dependent cytolysin (CDC) superfamily and shares functional similarities with perforin-like proteins. C9 exists in a monomeric state in plasma and circulates at a concentration of approximately 60 µg/mL. Upon activation, it undergoes significant conformational changes to facilitate membrane insertion and polymerization, forming the pore structure of the MAC.
Table 1 Function of complement component 9.
|
Function
|
Description
|
Process
|
|
MAC Formation
|
The primary role of Complement C9 lies in its contribution to MAC assembly, which targets and eliminates pathogens.
|
-
Activation of Complement C5 to C5b through cleavage by the C5 convertase
-
Sequential binding of C5b to Complement C6, C7, and C8, forming a complex that anchors to the target cell membrane
-
Recruitment and polymerization of multiple C9 molecules around C5b-8, forming a transmembrane pore
|
|
Synergy with Complement C5 to C8
|
Complement C9 works in concert with Complement C5 to C9 components to exert its cytolytic effects. This synergy ensures precision and efficiency in targeting pathogenic cells without harming host cells.
|
-
C5b-8 initiates the assembly.
-
C9 completes the process by creating a pore with an internal diameter of ~10 nm.
|
|
Immune Regulation
|
Complement C9 indirectly influences immune responses by promoting the clearance of immune complexes and cellular debris.
|
-
Trigger pro-inflammatory signaling pathways in MAC formation
-
Enhance leukocyte recruitment and cytokine release
|
In general, complement component 9 represents a crucial element in the immune system's arsenal against pathogens. Complement C9 exhibits several critical functions in immune defense.
-
Formation of transmembrane channels in target cells, leading to osmotic lysis
-
Coordination with complement C5-C8 proteins to complete the MAC assembly
-
Enhancement of cellular immune responses through the release of inflammatory mediators
-
Participation in the clearance of pathogenic microorganisms and damaged cells
Therefore, deficiencies or dysfunctions in complement component 9 can lead to various pathological conditions.
What is Complement Component 9 Deficiency?
C9 deficiency is a rare immunological condition characterized by the absence or dysfunction of the C9 protein, which is an integral part of the complement system. This deficiency disrupts the formation of the MAC, a critical component of innate immunity responsible for lysing pathogens and maintaining immune homeostasis.
C9 deficiency is often inherited in an autosomal recessive pattern and is most commonly associated with mutations in the C9 gene, located on chromosome 5. These mutations can result in:
-
Complete deficiency: Total absence of functional C9 protein.
-
Partial deficiency: Reduced levels of functional C9, leading to diminished MAC formation.
While many individuals with C9 deficiency remain asymptomatic, the condition increases susceptibility to certain infections.
Table 2 Deficiencies or dysfunctions in C9 can lead to various pathological conditions.
|
Features
|
Pathological Conditions
|
|
Increased Risk of Invasive Infections
|
-
Increased susceptibility to Neisseria infections
-
Impaired defense against gram-negative bacteria
-
Severe and recurrent infections, especially in childhood or early adulthood
|
|
Variable Immune Responses
|
-
Potential involvement in neurodegenerative disorders
-
Association with certain autoimmune conditions
|
|
Silent Nature
|
|
Diagnosis of C9 Deficiency
The laboratory workflow for evaluating C9 deficiencies usually includes:
-
Complement functional assays - particularly CH50 testing, which typically shows reduced or absent activity despite normal alternative pathway function.
-
Protein quantification - specific quantification of C9 protein levels using immunological methods such as ELISA or Western blotting.
-
Genetic testing - genetic sequencing to identify specific mutations in the C9 gene, providing a definitive diagnosis and valuable information.
Complement C9 in Immunology and Therapeutics
Complement C9 is a single-chain glycoprotein that plays a fundamental role in the terminal pathway of complement activation . Recent advancements in therapeutic approaches targeting C9 have opened new frontiers in treating complement-mediated disorders, presenting significant opportunities for pharmaceutical development and clinical applications.
Emerging therapies focus on modulating C9 activity to control complement dysregulation in a variety of diseases. Therapeutic approaches include:
-
Inhibition of MAC formation: preventing excessive C9 polymerization to mitigate tissue damage in conditions such as PNH.
-
Gene therapy: to correct C9 deficiency by CRISPR-Cas9 or viral vector-based interventions.
The terminal pathway culminates in the assembly of MAC (C5b-C9), which forms transmembrane pores, leading to osmotic lysis of targeted cells. This is particularly effective against Gram-negative bacteria.
Table 3 C9 in drug development.
|
Therapeutic Approaches
|
Descriptions
|
Advantages
|
|
Monoclonal Antibodies
|
Monoclonal antibodies target earlier steps in the complement cascade, indirectly reducing C9 activity or aim for direct inhibition of C9 to achieve more precise control.
|
-
High specificity
-
Reduced off-target effects
|
|
Small Molecule Inhibitors
|
Innovative small molecules are being developed to block C9 interactions with the C5b-C8 complex, offering potential treatments for diseases like myasthenia gravis and neuromyelitis optica spectrum disorder (NMOSD).
|
-
Oral bioavailability
-
Ttissue penetration
|
|
Recombinant C9
|
Recombinant C9 proteins are being explored for ex vivo diagnostic applications and as supplements for individuals with genetic deficiencies.
|
-
Long-term regulation of C9 levels
|
The field of C9 therapeutics continues to evolve, several pharmaceutical companies have initiated clinical trials evaluating C9-targeted therapeutics. Recent technological advances in protein engineering and drug delivery systems have enabled the development of more effective C9-targeted therapeutics.
Creative Biolabs offers a full range of complement component C9-related services and products, including:
If you want more information, please feel free to contact us.
Resources
References
-
Spicer, Bradley A., et al. "The first transmembrane region of complement component-9 acts as a brake on its self-assembly." Nature Communications 9.1 (2018): 3266.
-
Parsons, Edward S., et al. "Single-molecule kinetics of pore assembly by the membrane attack complex." Nature communications 10.1 (2019): 2066.
-
under Open Access license CC BY 4.0, without modification
For Research Use Only.
Related Sections:

 Fig.1 Structure of complement component 9.1,3
Fig.1 Structure of complement component 9.1,3



