Exosome-Based Vaccine against Cancer
Exosome-based delivery vectors as a non-cellular intervention strategy for vaccines that carry multiple active molecules and immune adjuvants to induce immune response and cancer cell killing. With an advanced exosome research platform, Creative Biolabs can provide high quality exosome-based vaccine development services to help clients advance cancer intervention research.
Mechanisms of Vaccine against Cancer
Engineered vaccines have facilitated research into interventions and treatments for cancer, providing new insights into treatment and prevention. By targeting tumor-specific antigens and highly expressed tumor-associated antigens, vaccines against cancer are able to induce a CD4+ / CD8+ effector T cell response, thereby restoring the effective suppressive effect of the immune system on cancer cells. In addition, unlike the preventive function of other vaccines, anti-cancer vaccines play a primarily therapeutic intervention role. It stimulates the immune response by enhancing antigen presentation, thus promoting the clearance of cancer cells. For example, mature dendritic cells (DCs), which specialize in the processing and presentation of antigens, are key candidates for vaccine development. However, due to the enhanced immunosuppressive effects of the tumor microenvironment, cell-based vaccines have demonstrated uncertainty in their anticancer efficacy by retaining only a low capacity to uptake antigens and activate T cells in clinical trials. Therefore, it is necessary to lift immunosuppression by combining immune adjuvants and optimizing adjuvant carriers.
 Fig.1 Mechanisms and components of an effective cancer vaccine. (Hu, 2018)
Fig.1 Mechanisms and components of an effective cancer vaccine. (Hu, 2018)
Exosome-based Vaccine against Cancer
A vaccine strategy based on multiple cell-derived exosomes is feasible, with good stability and targeting characteristics, and its sources include B lymphocytes, macrophages, DCs, etc. B lymphocyte-derived exosomes present antigen to CD4+ T cells via MHC II on their surface, induce clonal expansion and differentiation of multiple effector T cells, and promote secretion of anti-cancer such as IL-6, TNF-α. Macrophages secrete exosomes carrying pathogen-associated antigens, which play a role in promoting the maturation of DCs and the release of pro-inflammatory factors. Of particular importance, DCs-derived exosomes (DEXs), enriched in active molecules such as MHC I, MHC II, CD86 and heat shock proteins, can activate both CD4+ and CD8+ T cells and increase the secretion of IFN-γ and IL-2, thereby inducing more potent anti-cancer activity. In addition, miRNAs containing a wide range of regulatory functions and proteins that activate innate immunity (e.g., promote NK cell value-added and activation) were also detected, resulting in DEXs with stronger resistance to immunosuppression of the tumor microenvironment. In conclusion, the use of adjuvant carriers of exosomes with important effects on antigen presentation is promising as a basic idea for the development of cancer vaccines.
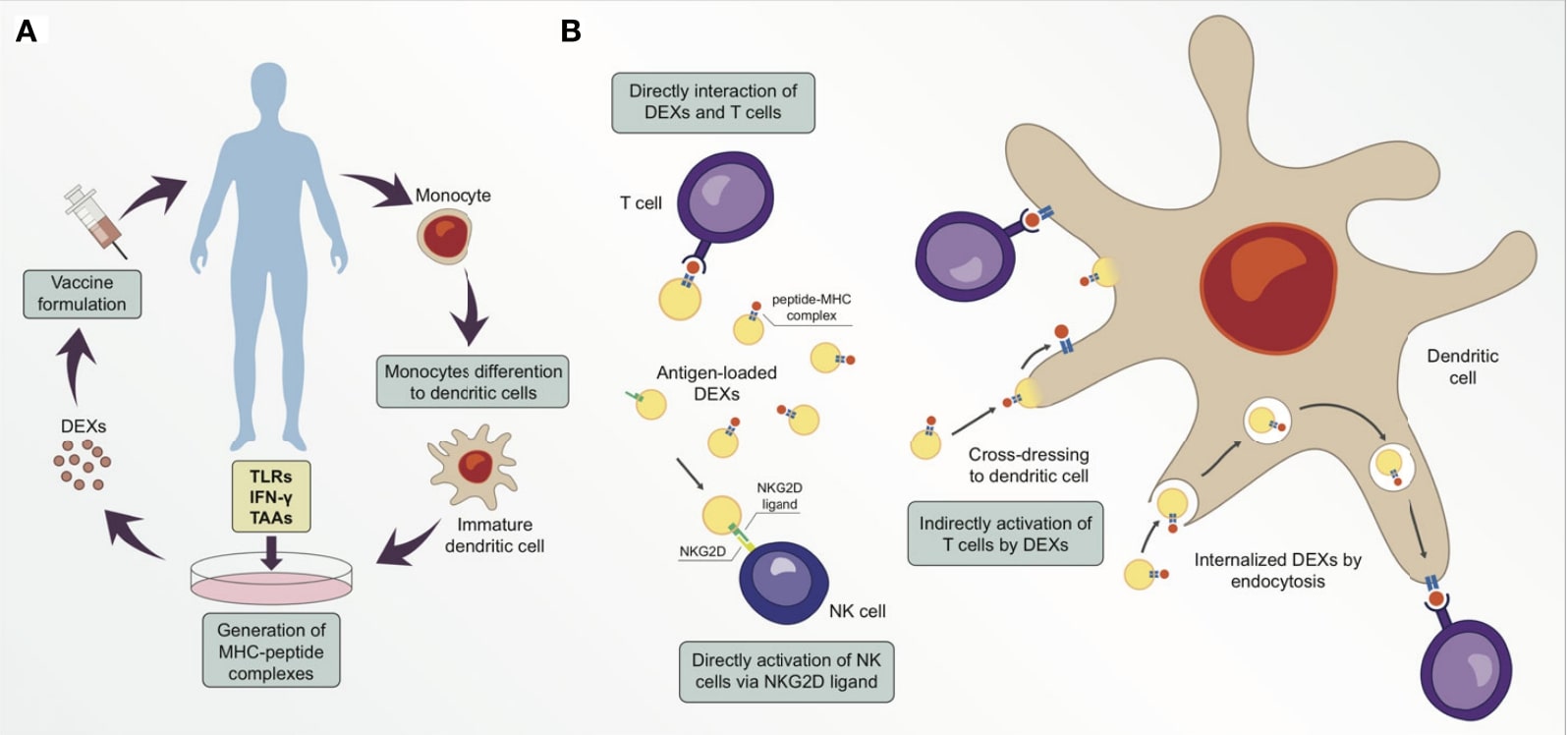 Fig.2 DEXs can be used as anti-cancer vaccines. (Santos & Almeida, 2021)
Fig.2 DEXs can be used as anti-cancer vaccines. (Santos & Almeida, 2021)
Advantages of Exosome-based Vaccines
The development of conventional engineered vaccines based on immune cells not only has extremely high standardized yield requirements, but also is difficult to implement in large populations, which requires high cost support. Viable exosomal vaccines are capable of delivering multiple immunomodulatory proteins, nucleic acids and lipids to nearby or distant target cells and target organs due to their structural properties of lipid vesicles, which exert a higher level of intervention. In addition, exosome-based vaccines are less cost-effective and easier to administer due to their high stability and biocompatibility than cell-based vaccines, which are difficult to standardize for generation and activity preservation.
The development of exosome vaccines of multiple immune cell sources is important to slow down the progression and spread of cancer. Creative Biolabs has established a series of well-established research systems including exosome preparation, purification, and functional validation, and is able to provide clients with exosome-based vaccine research services related to resistance to cancer. Please contact us for more information.
References
-
Hu, Z.; et al. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018, 18(3): 168-182.
-
Santos, P.; Almeida, F. Exosome-based vaccines: History, current state, and clinical trials. Front Immunol. 2021, 12: 711565.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Mechanisms and components of an effective cancer vaccine. (Hu, 2018)
Fig.1 Mechanisms and components of an effective cancer vaccine. (Hu, 2018)
 Fig.2 DEXs can be used as anti-cancer vaccines. (Santos & Almeida, 2021)
Fig.2 DEXs can be used as anti-cancer vaccines. (Santos & Almeida, 2021)








