Therapeutic Exosomes for Alzheimer's Disease
Exosomes can induce physiological changes in target cells, which are achieved after being internalized by cells through membrane fusion, phagocytosis, macrophage phagocytosis, lipid raft-mediated internalization, and endocytosis involving cage and niche proteins. Researchers worldwide are using exosome-based studies to decipher disease mechanisms or as therapeutic drug development, especially in the field of neurodegenerative diseases. Creative Biolabs has accumulated extensive experience in scientific services in the field of exosome extraction, identification and functional validation to help and encourage clients to conduct Alzheimer's disease (AD)-related exosome research.
Pathogenesis of AD
Alzheimer's disease (AD) is a late-onset neurodegenerative disorder characterized by progressive memory and cognitive decline, impairment of normal activities, and ultimately a complete loss of individual autonomy. The presence of insoluble deposits in neurons, including median senile plaques (SPS) and neurogenic fibrillary tangles (NFTs), are two major histopathological features of AD, with amyloid β protein (Aβ) and abnormal Tau protein as the main components, respectively. The process of formation of these toxic pathological proteins mainly involves the action of the γ-secretase complex and the abnormal phosphorylation process of the proteins. In conclusion, the formation of insoluble pathological protein aggregates induces protein degradation dysfunction of lysosomes, cellular inflammatory response and neurotoxicity, which ultimately leads to neuronal cell death and neurodegeneration. Meanwhile, the dissemination of toxic protein aggregates along the synapse further expands the AD lesion area and deepens the deterioration of AD.
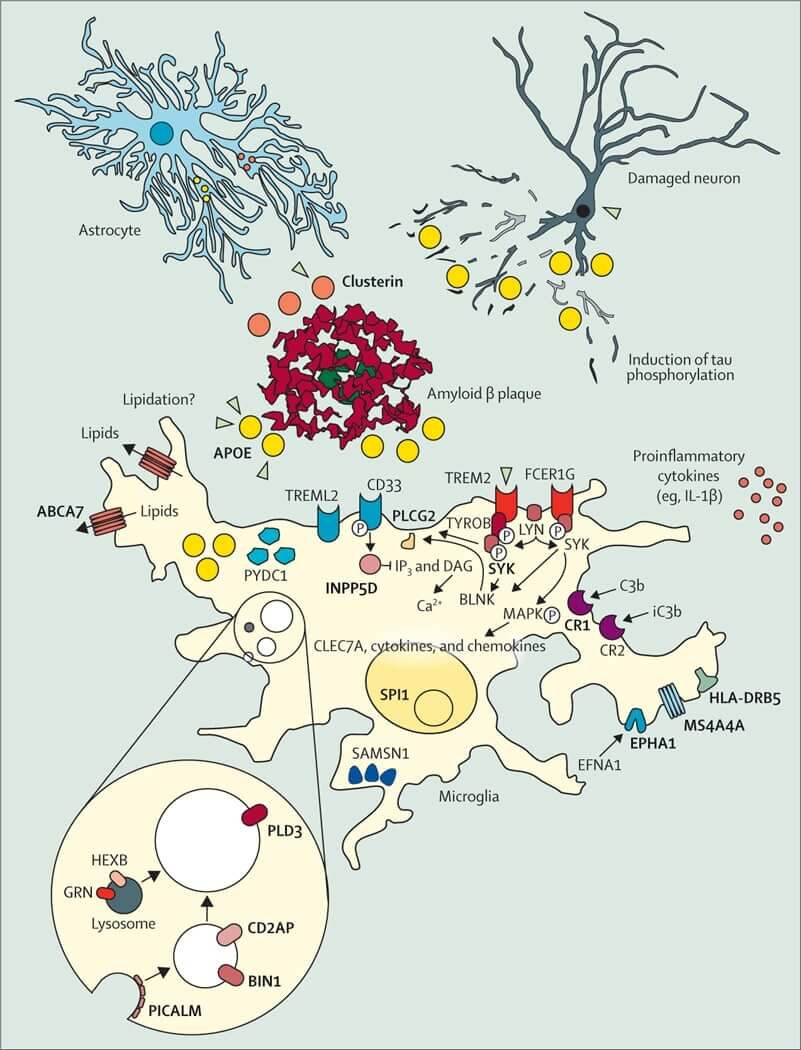 Fig.1 The cellular phase of AD. (Scheltens, 2021)
Fig.1 The cellular phase of AD. (Scheltens, 2021)
Exosomes Rely on Natural Advantages for AD Treatment
Exosomes are one of the nanoscale vesicle subtypes secreted by early endosomes of cells such as plants, mammals and microorganisms. Over the past decades, exosome-based therapeutic agents have gained much attention in the study of brain diseases such as AD. For example, exosomal surface protein and prion protein (PRION)-mediated exosome injection can eliminate synaptic plasticity dysfunction caused by Aβ by isolating Aβ and delaying the aggregation and deposition of insoluble pathological fibers. In this process, Aβ is taken up and cleared by microglia with the help of exosomes, effectively reducing the toxic damage caused by Aβ. Exosomes secreted from human umbilical cord mesenchymal stem cells and adipose stem cells showed therapeutic potential in AD transgenic mouse models, reducing the levels of pro-inflammatory cytokines and upregulating the levels of anti-inflammatory cytokines to avoid inflammatory damage. At the same time, the activity of enkephalinase (NEP) and insulin-degrading enzyme (IDE), two Aβ-degrading enzymes, was restored and a reduction in the number of SPs in the cerebral cortex and hippocampus was seen on histopathology. In the AD mouse model treated with stem cell-derived exosomes, there was an increase in neurogenesis and axonal growth that could be clearly detected, and the mice showed some improvement in memory, learning and cognition. In addition, endothelin-converting enzyme 1/2 and metalloproteinases have been shown to have the ability to degrade Aβ and have been found to be present in exosomes.
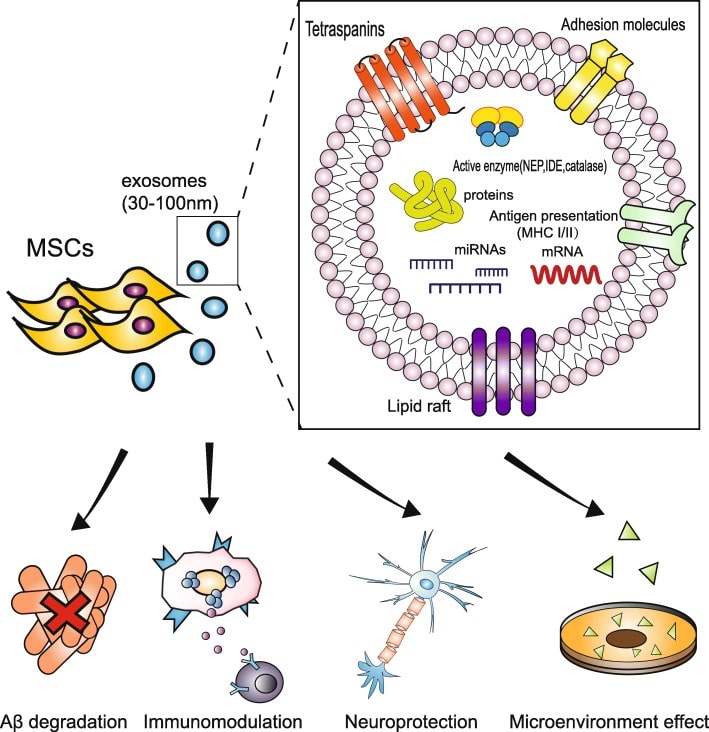 Fig.2 The cargo and therapeutic role of mesenchymal stem cell-derived exosome in AD. (Guo, 2020)
Fig.2 The cargo and therapeutic role of mesenchymal stem cell-derived exosome in AD. (Guo, 2020)
Exosomes as Drug Delivery Vehicles for AD Treatment
In addition to the therapeutic effects of exosomes in AD due to their own origin, the structural advantage of lipid vesicles in exosomes facilitates their delivery of proteins, nucleic acids and drugs across the blood-brain barrier (BBB) with good specificity to the central nervous system. This fact enhances the therapeutic potential of exosome-delivered drugs in AD. For example, exosomal delivery of siRNA targeting BACE1 reduces the toxic Aβ produced by cleavage of amyloid precursor protein (APP) by the synthetic BACE1 enzyme, which is one of the promising gene therapy strategies. In parallel, the possible molecular mechanism by which exosomes carrying curcumin exert pathological protein scavenging effects has been supported by evidence from preclinical experiments. Therefore, there has been a lot of very worthwhile research work to be done in the field of exosome-based development of AD therapeutics.
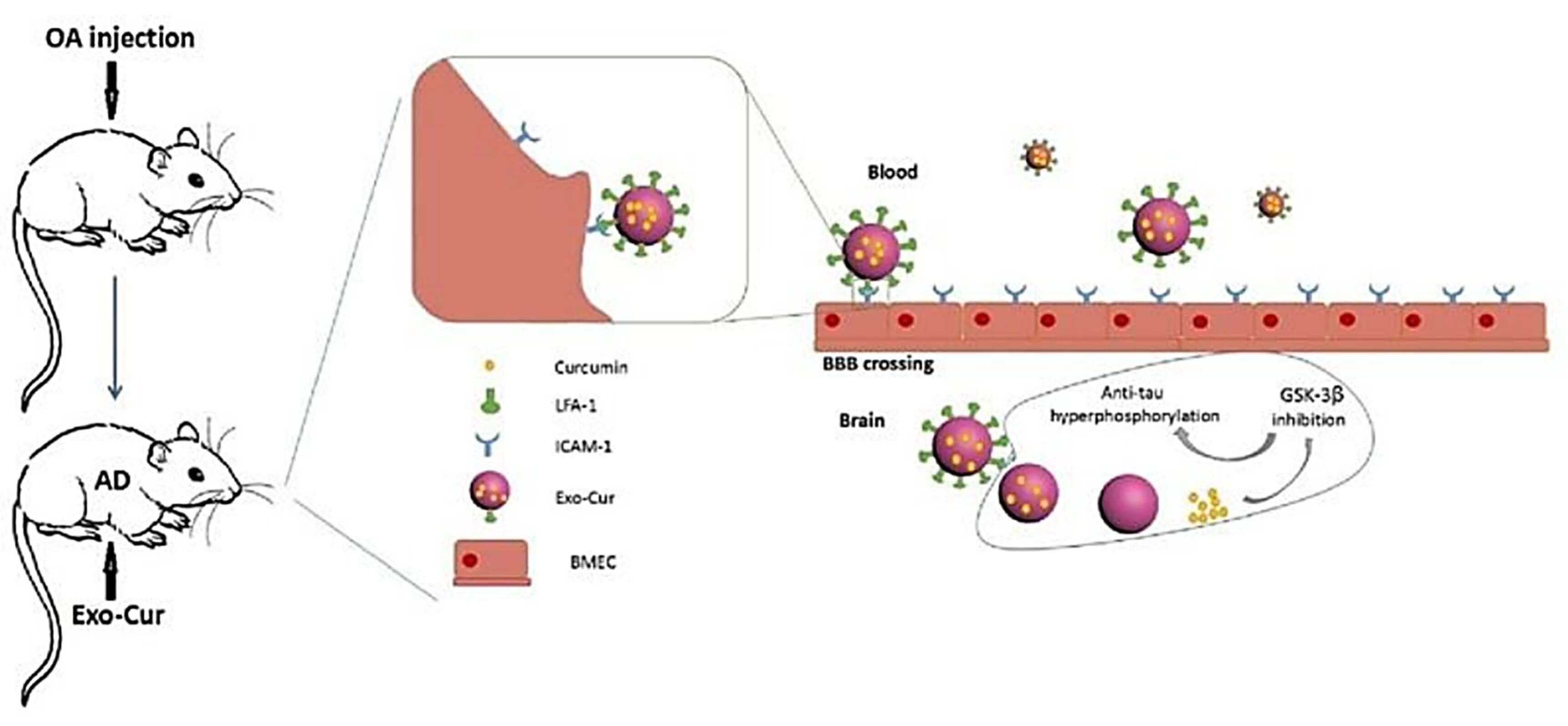 Fig.3 Curcumin-primed exosomes potently ameliorate cognitive function in AD mice. (Wang, 2019)
Fig.3 Curcumin-primed exosomes potently ameliorate cognitive function in AD mice. (Wang, 2019)
Exosomes act as mediators that can regulate the physiological state of nearby or distant target cells through processes such as release, transport, capture and internalization. Multiple lines of evidence reinforce the importance of exosome-based drug development in the treatment of neurological diseases such as AD. Creative Biolabs is confident to provide customized services in exosome extraction, identification and development of drugs targeting AD therapeutics to meet our clients' research needs. Please contact us with your needs.
References
-
Scheltens, P.; et al. Alzheimer's disease. Lancet. 2021, 397(10284): 1577-1590.
-
Guo, M.; et al. Mesenchymal stem cell-derived exosome: a promising alternative in the therapy of Alzheimer's disease. Alzheimers Res Ther. 2020, 12(1): 109.
-
Wang, H.; et al. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3β pathway. Nanoscale. 2019, 11(15): 7481-7496.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 The cellular phase of AD. (Scheltens, 2021)
Fig.1 The cellular phase of AD. (Scheltens, 2021)
 Fig.2 The cargo and therapeutic role of mesenchymal stem cell-derived exosome in AD. (Guo, 2020)
Fig.2 The cargo and therapeutic role of mesenchymal stem cell-derived exosome in AD. (Guo, 2020)
 Fig.3 Curcumin-primed exosomes potently ameliorate cognitive function in AD mice. (Wang, 2019)
Fig.3 Curcumin-primed exosomes potently ameliorate cognitive function in AD mice. (Wang, 2019)








