Kits
Advantages Products Publish Data FAQs Resources
Advantages of Kits for Preparing Liposomes or LNPs
Liposomes are spherical vesicles composed of phospholipid bilayers that can encapsulate both hydrophilic and hydrophobic drugs. Lipid nanoparticles (LNPs) are lipid-based nanocarriers primarily used for encapsulating nucleic acid drugs (such as mRNA) and certain small molecule drugs. Kits designed for the preparation of liposomes and LNPs can enhance drug delivery efficiency and offer the following advantages:
-
Standardization and Consistency: The standardized protocols and formulations ensure consistent results across experiments.
-
Simplified Procedure: are equipped with all necessary materials for liposome or LNP preparation, streamlining the process.
-
Reproducibility: The materials and procedures in the kits are optimized and validated, leading to higher reproducibility.
-
Time Efficiency: Liposome/LNP preparation can be completed in a short timeframe.
-
Convenient Storage and Transport: The kits provide lyophilized lipids that are suitable for long-term storage.
Creative Biolabs' Kit Products
As a supplier focused on lipid-based delivery systems, Creative Biolabs has developed optimized formulations and processes to create kits that efficiently prepare liposomes or lipid nanoparticles (LNPs) in a short time frame. These kits are designed for the direct preparation of liposomes or LNPs and offer excellent reproducibility. You can choose the appropriate kit based on the active substances you wish to encapsulate or the lipids of interest. Once you contact us, you will receive professional technical support and consulting services.
Please refer to our detailed list of kit products:
| Cat |
Product name |
Lipid Composition |
Data sheet |
MSDS |
Inquiry |
| LDLY-0724-LD136 |
Liposome Preparation Kit |
Cholesterol, Egg PC, Stearylamine |

|

|
Inquiry
|
| LDLY-0724-LD137 |
NHS-Immunoliposomes Preparation Kit (PEGylated) |
HSPC, Cholesterol, DSPE-PEG (2000)-NHS, DSPE-PEG (2000) |

|

|
Inquiry
|
| LDLY-0724-LD138 |
LNP Preparation Kit (SM102) |
SM102, Cholesterol, DSPC, DMG-PEG (2000) |

|

|
Inquiry
|
| LDLY-0724-LD139 |
LNP Preparation Kit (DLin-MC3-DMA) |
DLin-MC3-DMA, Cholesterol, DSPC, DMG-PEG (2000) |

|

|
Inquiry
|
| LDLY-0724-LD140 |
LNP Preparation Kit (ALC-0315) |
ALC-0315, Cholesterol, DSPC, DMG-PEG (2000) |

|

|
Inquiry
|
| LDLY-0724-LD141 |
LNP Preparation Kit (cKK-E12) |
cKK-E12, Cholesterol, DSPC, DMG-PEG (2000) |

|

|
Inquiry
|
Publish Data
Optimizing mRNA-Loaded Lipid Nanoparticles as a Potential Tool for Protein-Replacement Therapy
Author: Gambaro, Rocío, et al.
This study explored the impact of lipid nanoparticles (LNPs) formulated with different lipid mixtures on mRNA delivery efficiency. Researchers utilized microfluidics to prepare four types of LNPs (LM1, LM2, LM3, and LM4) encapsulating EGFP mRNA, based on various lipid compositions. Subsequently, they evaluate the transfection efficiencies of a commercial formulation (GV) and the four LNPs in delivering EGFP mRNA to HepG2 cells. The results from the EGFP+ cells (figure a) indicated that all LNPs demonstrated high transfection efficiencies, with percentages of 93%, 87%, 96%, 87%, and 98% for GV, LM1, LM2, LM3, and LM4, respectively. Fluorescence microscopy results (figure b and c) revealed that the mean fluorescence intensity (MFI) of the positive control group was significantly higher than that of the other groups. In comparison, LM4 (DLin-MC3-DMA/DSPC/Cholesterol/ALC-0159) exhibited a higher MFI than GV, LM1, LM2, and LM3. Overall, the results suggest that the LNPs effectively delivered EGFP-encoding mRNA to HepG2 cells.
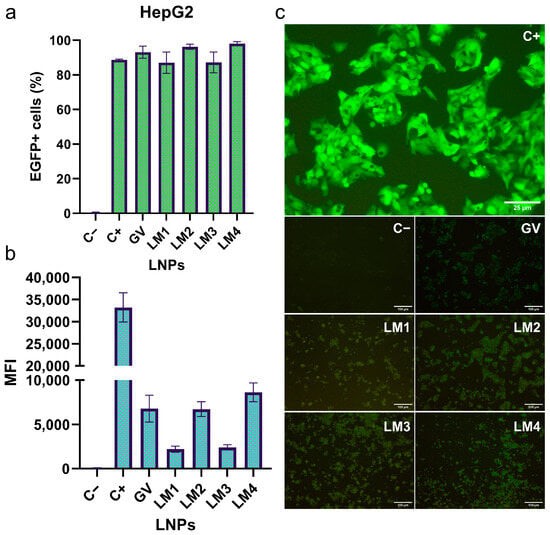 Fig.1 Efficiency of different LNP formulations in transfecting EGFP mRNA into HepG2 cells.1,2
Fig.1 Efficiency of different LNP formulations in transfecting EGFP mRNA into HepG2 cells.1,2
FAQs
What are the main components of these kits?
These kits are composed of lyophilized lipids, such as phospholipids, cholesterol, and other lipids, which are used to form liposomes/LNPs.
Do the kits include detailed operating instructions?
Yes, the kits come with a detailed operation manual and precautions to ensure the smooth preparation of liposomes/LNPs.
How do I choose the appropriate kit?
You can select the suitable kit based on the properties of the material to be encapsulated in order to achieve optimal encapsulation efficiency.
Are the kits suitable for large-scale production?
These kits are primarily designed for laboratory-scale applications. If you need production on a large scale, our bulk production services can fulfill your requirements.
Do these kits contain any toxic or harmful substances?
The materials in these kits are typically regarded as having low toxicity. However, it is essential to follow proper laboratory safety procedures.
Resources
References
-
Gambaro, Rocío, et al. "Optimizing mRNA-Loaded Lipid Nanoparticles as a Potential Tool for Protein-Replacement Therapy." Pharmaceutics 16.6 (2024): 771.
-
under Open Access license CC BY 4.0, without modification.

For Research Use Only. Not For Clinical Use

 Fig.1 Efficiency of different LNP formulations in transfecting EGFP mRNA into HepG2 cells.1,2
Fig.1 Efficiency of different LNP formulations in transfecting EGFP mRNA into HepG2 cells.1,2



 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical Use
