The phage display method serves as a potent molecular approach which connects phenotypic expression to genotypic information through bacteriophages displaying peptides and proteins on their surfaces. The phage display system transformed ligand selection methods and stands as an essential tool in drug discovery research along with immunology and protein engineering. Since its invention by George P. Smith in 1985 phage display technology developed into an essential method used for screening combinatorial libraries and investigating target-ligand interactions.
| Era | Sequencing Method | Limitations | Capabilities Improved |
| 1990s | Sanger Sequencing | Low throughput, labor-intensive | Individual clone analysis |
| 2000s | Capillary Electrophoresis | Moderate throughput | Manual screening of enriched pools |
| 2010s–Present | Next Generation Sequencing | Ultra-high throughput | Quantitative profiling of millions of sequences |
High-resolution sequencing bridges the gap between diversity exploration and rational selection.
Next Generation Sequencing (NGS), also known as high-throughput sequencing, brings a fundamental change to the analysis of phage display libraries. Next Generation Sequencing allows scientists to study millions of phage-displayed sequences simultaneously during one run and delivers unmatched depth and scope compared to traditional Sanger sequencing which analyzes only tens to hundreds of clones.
NGS technology sequences the variable regions found in phage display such as antibody CDRs and random peptides presented on phage coat proteins. The technique provides detailed analysis of complete libraries and the ability to track selection rounds through quantitative and data-focused comprehensive evaluation.
NGS in phage display is not just a technological improvement—it is a critical enabler of modern ligand discovery, immune repertoire analysis, and high-confidence binder enrichment.
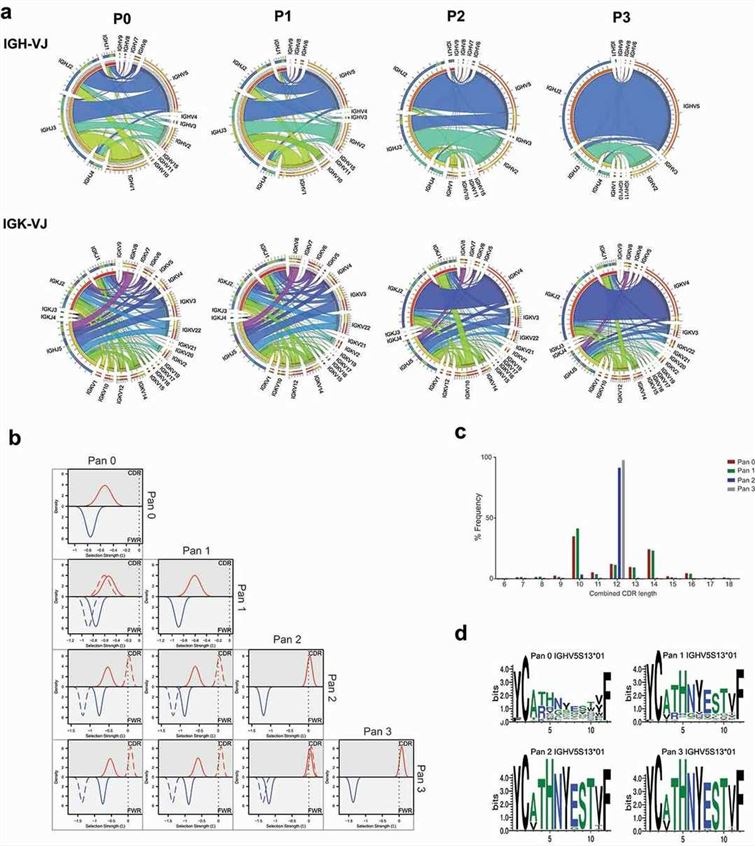 Fig. 1 Analysis of CD160 library diversity after phage display enrichment.1
Fig. 1 Analysis of CD160 library diversity after phage display enrichment.1
By sequencing phage pools before and after each round of panning, NGS allows precise tracking of enrichment kinetics, revealing which sequences increase in frequency over time and are likely to represent high-affinity binders.
NGS delivers not only the identity but also the relative abundance of each sequence. This allows researchers to distinguish between dominant binders and background noise, making hit selection more robust and evidence-based.
With millions of reads, NGS can provide a detailed snapshot of the diversity landscape within a library. Rare clones that may hold therapeutic value but are often lost in traditional screens can now be identified and tracked.
Real-time insights from NGS enable dynamic adjustment of panning conditions, such as increasing selection stringency or switching target presentation formats, to steer the enrichment towards optimal candidates.
Each sequencing platform offers unique strengths. The choice of platform depends on the library type (e.g., scFv, Fab, peptide), required read length, throughput, and project timeline.
| Platform | Read Length | Sequencing Depth | Turnaround Time | Best Used For |
| Illumina MiSeq/NextSeq | 2×150 bp / 2×300 bp | Up to 800M reads | 1–3 days | High-resolution antibody or peptide libraries |
| Ion Torrent S5 | 200–400 bp | Up to 80M reads | <1 day | Fast exploratory sequencing |
| PacBio Sequel II | >10,000 bp | Lower throughput | 3–5 days | Full-length scFv/Fab profiling |
| Oxford Nanopore | Variable | Real-time sequencing | <1 day | Field-ready or on-demand sequencing |
Note: Illumina remains the gold standard for short-read sequencing in phage display due to its high fidelity and data quality.
Deep sequencing refers to the ultra-high-throughput analysis of nucleic acid populations, delivering vast read depth and coverage that enables the exhaustive characterization of phage display libraries. In contrast to conventional sequencing methods that profile hundreds of clones, deep sequencing—especially when performed via NGS platforms like Illumina or Ion Torrent—can analyze millions to billions of unique sequences per sample, offering unprecedented insights into:
 Fig. 2 Overview of strategy to generate antibodies from deep-sequenced scFv libraries.1
Fig. 2 Overview of strategy to generate antibodies from deep-sequenced scFv libraries.1
Deep sequencing has become the gold standard for library assessment, candidate prioritization, and evolutionary tracking in both antibody and peptide phage display workflows.
| Feature | Sanger Sequencing | Deep Sequencing (NGS) |
| Throughput | 96–384 sequences per run | Millions of reads per run |
| Sensitivity to Minor Clones | Low | High – detects rare binders (<0.01%) |
| Quantitative Clonal Abundance | No | Yes – frequency distribution per sequence |
| Round-to-Round Enrichment Tracking | Manual, slow | Automated, real-time resolution |
| Motif and Family Group Identification | Limited | Robust clustering and alignment possible |
| Time and Cost Efficiency | Expensive per sequence | Low cost per million sequences |
Each stage of the workflow demands high stringency in sample preparation, particularly for PCR amplification steps which can introduce bias. Unique molecular identifiers (UMIs) are increasingly used to deconvolute true clone abundance from PCR duplicates.
Phage-Displayed Immunoprecipitation Sequencing (PhIP-Seq) merges the comprehensive qualities of phage display with immunoprecipitation sensitivity and next-generation sequencing depth. PhIP-Seq facilitates high-throughput profiling of immune repertoires against substantial peptide libraries that are either synthetic or natural by systematically investigating antibody–epitope interactions.
PhIP-Seq which began as a technique for profiling autoantibodies now serves multiple research areas including:
PhIP-Seq provides quantitative and multiplexed high-dimensional analysis of antibody reactivity landscapes with ultra-deep sequencing which traditional methods like ELISA or Western blot do not offer.
Step 1: Library Design
Libraries typically consist of overlapping 15–90-mer peptides, spanning entire proteomes (e.g., human, viral), and codon-optimized inserts cloned into M13 phage systems.
Step 2–3: Antibody Binding & Immunoprecipitation
Samples (e.g., patient serum or monoclonal antibodies) are incubated with the phage library. Antibody–phage complexes are pulled down using protein A/G magnetic beads.
Step 4–6: Sequencing & Analysis
Phage DNA corresponding to bound peptides is PCR-amplified. NGS is used to quantify enrichment of each peptide. Enriched sequences represent targeted epitopes.
PhIP-Seq technology provides an opportunity to find new autoantigens in conditions such as Systemic lupus erythematosus (SLE), Sjögren's syndrome, Multiple sclerosis, and Type 1 diabetes.
When researchers perform screenings against synthetic human peptidomes they discover patient-specific reactivity profiles that could result in new biomarkers or personalized therapeutic targets.
Characterize serological response to pathogens (e.g., SARS-CoV-2, Influenza, HIV). Distinguish between past infection, vaccination, or cross-reactivity. Identify neutralizing epitope clusters for rational vaccine design.
PhIP-Seq can systematically map linear B-cell epitopes across proteomes, regions of epitope conservation vs. variability, and informing the design of next-generation vaccines (e.g., pan-coronavirus).
| Technique | Throughput | Resolution | Multiplexing | Cost per Sample | Quantitative |
| ELISA | Low | Single antigen | No | High | Semi-quantitative |
| Peptide Arrays | Medium | ~10,000 spots | Moderate | Moderate | No |
| Mass Spectrometry | High | PTM-sensitive | Moderate | High | Yes |
| PhIP-Seq | Very High | Proteome-wide | Yes | Low | Yes |
PhIP-Seq provides combinatorial scalability with nucleotide-level resolution, making it especially suited for:
Phage display sequencing, especially with the integration of next-generation and deep sequencing technologies, has redefined the landscape of molecular interaction discovery. At Creative Biolabs, our commitment to innovation ensures clients can harness the full power of library-based discovery with scientific precision and commercial excellence.
Learn more about Creative Biolabs premade phage display library ready-to-use kits and premade phage display services:
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
