At present, animal models used in preclinical research mainly include small animal models with high repeatability and low cost, such as inbred mice. However, many small animal models cannot accurately predict human response due to their different genetic backgrounds and cannot eliminate the influence of species specificity on preclinical research, so many preclinical animal experimental results are very different from the human clinical response.
Some studies have shown that major histocompatibility complex (MHC) plays important roles in biological defense system by expressing a variety of antigen peptides and inducing T cell immune response, and the genetic difference of MHC molecules among species is one of the important factors leading to this difference. Therefore, it can be considered to replace animal MHC molecules with human leukocyte antigen (HLA) molecules in the experimental animals, so as to effectively improve the accuracy of animal models in predicting human immune response. Creative Biolabs makes full use of this difference and cooperates with partners to develop the HLA transgenic mouse model. With the unique SIAT® platform, we can provide a one-stop in vivo immunogenicity analysis service.
 Fig.1 Immunogenicity assessment workflow.
Fig.1 Immunogenicity assessment workflow.
T cell receptors (TCRs) are the antigen recognition mechanism on the surface of T lymphocyte. TCR forms homologous interactions with antigens through MHC in mice, human HLA on the surface of somatic cells, and specialized antigen-presenting cells of the innate immune system. TCR recognition activates the downstream immune response, which produces strong adaptive immunity.
The transplantation of HLA into immunocompromised mice is an important new tool for understanding human-specific pathogens and their immune responses. Using the mouse model, we can find the specific haplotype T cell epitope, which provides a powerful evaluation method for immunogenicity analysis.
 Fig.2 Genetic humanization of individual targets or of entire portions of the immune system. (Zitvogel, 2016)
Fig.2 Genetic humanization of individual targets or of entire portions of the immune system. (Zitvogel, 2016)
Immunologic analysis in HLA transgenic mice as the most important step in the immunogenicity test, a large amount of literature research and information retrieval are needed in the early stage. This model that can replicate human antigen presentation with HLA epitope binding specificity will continue to be used in more and more ways. Traditional vaccine methods, including epitope identification and validation, have been extended to cancer vaccine research. The evaluation of the antitumor activity of the tumor vaccine can be carried out directly in HLA transgenic mice. In T cell immunotherapy, HLA transgenic mice have been used to identify the sequence of high-affinity TCR, so as to develop a new chimeric antigen receptor (CAR) T-cell therapy. With the increase of immunotherapy research, these tools will become more and more important.
In addition, an experiment has been applied to the evaluation of HLA transgenic mice to Graves' disease. Scientists combined the histocompatibility complex binding test with the immune and tolerance induction test of HLA transgenic mice to prepare epitopes (antigen processing independent epitopes) from the thyroid-stimulating hormone receptor (TSHR). When the HLA transgenic mice were injected, the mixture of two immunodominant epitopes was sufficient to inhibit the response of T cells and antibodies to TSHR without damaging the tolerance of the mice.
In terms of immunologic analysis, Creative Biolabs has rich experimental experience and advanced technology. If you have any requirements, please contact us.
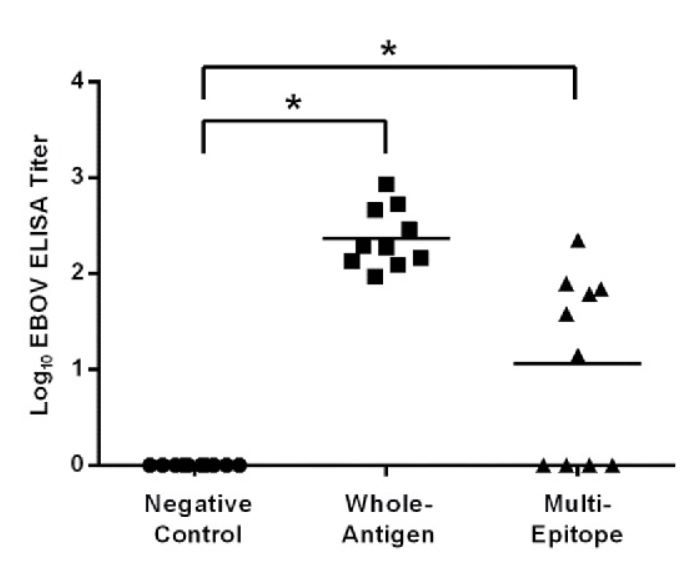 Fig. 3 Virus-specific antibody responses elicited in vaccinated HLA-DR3 mice. (Callie E. Bounds, 2017)
Fig. 3 Virus-specific antibody responses elicited in vaccinated HLA-DR3 mice. (Callie E. Bounds, 2017)
The research described in the article demonstrates the utility of an immunoinformatics-derived DNA vaccine encoding class II T cell epitopes from various viruses, which proved immunogenic in HLA transgenic mice. This study underscores the potential for using HLA transgenic mice to evaluate the immunogenicity of vaccines designed based on human immune responses, thereby bridging a crucial gap between preclinical studies and human trials. HLA transgenic mice were crucial for confirming the vaccine's ability to induce a human-like immune response, notably demonstrating significant cellular and humoral responses upon vaccination. This model system is particularly valuable for assessing vaccine efficacy before clinical trials, highlighting its role in preclinical vaccine development.
HLA transgenic mice are utilized to evaluate the immunogenicity of biotherapeutics and vaccines. By expressing human leukocyte antigen (HLA) molecules, these mice provide a model that more closely mimics the human immune response, enabling researchers to predict potential immunogenic reactions in humans more accurately.
Unlike standard mice, HLA transgenic mice are genetically modified to express specific HLA molecules that are found in humans. This modification allows them to present peptides and proteins in a way that mimics human immune mechanisms, offering more relevant data on how a drug might interact with the human immune system.
Common HLA alleles used in transgenic models include HLA-DR, HLA-DQ, and HLA-A2 among others. These alleles are selected based on their prevalence in the human population and their known roles in drug-related immune responses, ensuring broad applicability of the results.
While HLA transgenic mice are excellent tools for predicting T-cell mediated immune responses due to their expression of human HLA molecules, they may not fully predict other immune responses, such as those mediated by antibodies or innate immune cells. Combining their use with other models can provide a comprehensive immunogenicity assessment.
One limitation is that while they express human HLA molecules, other aspects of their immune system remain murine. This can affect the interaction between cells and cytokines, potentially leading to differences in immune response compared to humans. Additionally, these models can be expensive and require specialized breeding and handling.
These mice are developed through genetic engineering techniques where genes encoding human HLA molecules are inserted into the mouse genome. This process often involves the use of vectors and promoters to ensure the correct expression of the HLA genes in appropriate tissues.
These mice are primarily used in preclinical studies to assess the immunogenic potential of therapeutic proteins, vaccines, and other biologics. Their use is crucial in drug development for autoimmune diseases, cancer, and infectious diseases where immune response plays a significant role.
Results from studies using HLA transgenic mice can significantly influence the development pathway of a biotherapeutic. Data on immunogenicity can lead to modifications in the drug's molecular structure, dosing regimens, or delivery methods to minimize adverse immune reactions in humans.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
