Creative Biolabs offers mammalian two-hybrid service, which allows rapid and convenient analysis of protein-protein interactions in transfected mammalian cells. Compared with the yeast two-hybrid system, the mammalian two-hybrid system provides the milieu for the bona fide posttranslational modification and localization of most eukaryotic proteins and, therefore, should be a better choice to study proteins of a mammalian origin.
Two-hybrid systems are extremely powerful methods for detecting protein-protein interactions in vivo. However, a major drawback of testing protein-protein interactions in a heterologous system such as the yeast is that interactions may depend on certain posttranslational modifications such as disulfide bridge formation, glycosylation, or phosphorylation, which may not occur properly or at all in the yeast system. In addition, since the fusion proteins in the two-hybrid system must be targeted to the nucleus, extracellular proteins or proteins with stronger targeting signals may be at a disadvantage. It has been shown that sub-domains of proteins may interact better than full length clones in yeast two-hybrid systems, perhaps due to the lack of certain folding restraints. What’s more, certain proteins may become toxic when expressed in the yeast two-hybrid system or that targeted to the nucleus may become toxic. Other proteins may degrade essential yeast proteins or proteins whose presence are required for the assay. Such genes may be counter-selected for during growth and may result in problems.
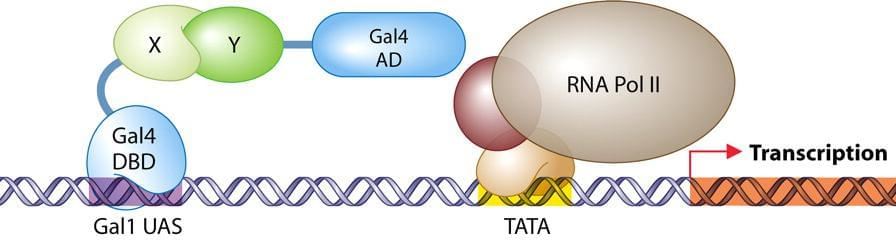
The mammalian two-hybrid system is a very powerful tool to investigate protein-protein interactions in terms of functional domains and identify potential binding ligands and partners of a protein. Creative Biolabs has adapted yeast two-hybrid system for use in mammalian cells. In our system, one protein (X) is fused to a DNA-binding domain interacts with a second protein (Y), which is fused to a transcriptional activation domain. A luciferase reporter gene was introduced as the readout of interaction between proteins X and Y.
One major advantage of our mammalian two-hybrid system over yeast systems is that mammalian protein interactions can be studied in an environment which is that is more similar to that in vivo. Our mammalian two-hybrid service can be applied in confirming suspected interactions between two proteins, identifying residues/domains involved in protein-protein interactions and identifying small molecules that affect protein-protein interactions, ect.
Other optional two-hybrid systems:
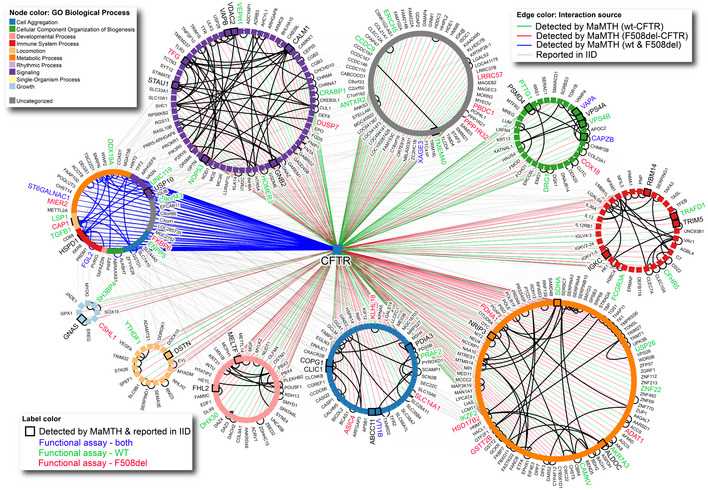 Fig. 3 MaMTH-HTS interactome for wt- and F508del-CFTR. (Sang Hyun Lim, 2022)
Fig. 3 MaMTH-HTS interactome for wt- and F508del-CFTR. (Sang Hyun Lim, 2022)
Cystic fibrosis transmembrane conductance regulator (CFTR) is the chloride and bicarbonate channel in the secretory epithelium, and it plays a key role in maintaining fluid homeostasis. The CFTR mutation is associated with cystic fibrosis (CF), and CF is the most common fatal autosomal recessive genetic disease in Caucasians. In this paper, the researchers reported the application of a high-throughput screening variant of mammalian membrane two-hybrid (MaMTH-HTS) to map protein-protein interactions between wild-type and mutant CFTR. It helps scientists better understand the effects of CF cells and identify new drug targets for patient-specific treatments. After functional verification and result analysis in a variety of disease models, they identified candidate proteins with potential roles in CFTR function/CF pathophysiology, including fibrinogen like 2 (FGL2). At the same time, the researchers demonstrated that the protein had a significant effect on the functional expression of CFTR in intestinal organs in patients.
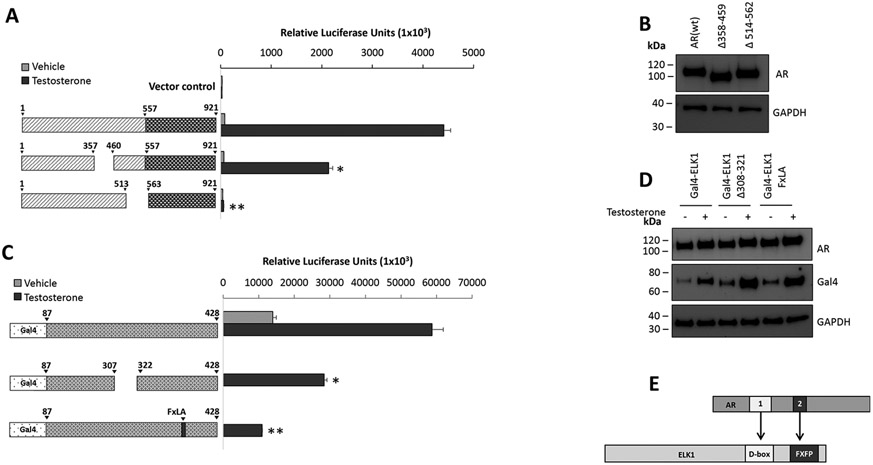 Fig. 4 Validation of putative ELK1 interacting segments using orthogonal and in situ assays. (Claire Soave, 2022)
Fig. 4 Validation of putative ELK1 interacting segments using orthogonal and in situ assays. (Claire Soave, 2022)
The growth of prostate cancer (PCa) requires the ETS domain transcription factor ELK1 to bind androgen receptor (AR) to chromatin to jointly activate cell proliferation genes. Destroying the ELK1-AR complex is an effective and potential method for the treatment of PCa. AR binds to ELK1 through its amino-terminal domain (A/B domain). Using the mammalian two-hybrid assay, the researchers mapped the amino acids in the A/B domain 358–457 and 514–557 peptides necessary for binding to ELK1 through functional experiments. The mapping data are verified by GST pulldown and BRET assays. Comparing the effects of interacting motifs/fragments in ELK1 and AR on the co-activation of ELK1 by AR showed that the binding mode of AR and ELK1 peptides was parallel. The growth of PCa cells was partially inhibited by the deletion of upstream fragments of AR, while the deletion of downstream fragments was almost completely inhibited. This study has determined that two peptides in AR can mediate the functional association between AR and two docking sites in ELK1. Therefore, in this study, the ELK1 recognition site of AR was identified to help scientists further study the structure of the ELK1-AR interaction and design small molecular drugs to destroy this interaction.
The mammalian two-hybrid assay is a modification of the yeast two-hybrid system, designed to study protein-protein interactions within the context of mammalian cells. This method allows researchers to identify and characterize interactions between two proteins in an environment that closely mimics in vivo conditions. In the M2H assay, one protein is fused to a DNA-binding domain, while the other protein is fused to a transcriptional activation domain. If the two proteins of interest interact, they bring these two domains close enough to drive the transcription of a reporter gene, typically encoding a fluorescent protein or an enzyme that confers antibiotic resistance. The activation of the reporter gene serves as an indicator of the interaction between the proteins under study.
Advantages:
Relevance: The assay is performed in mammalian cells, which makes the results more relevant to physiological conditions compared to other systems like yeast.
Versatility: It can be used with any pair of proteins of interest, as long as they can be expressed in the chosen mammalian cell line.
Sensitivity: M2H can detect even weak interactions, which might be missed by other methods like co-immunoprecipitation.
Limitations:
Context dependence: The interaction is observed only within the nucleus of the cell, which may not reflect interactions that occur in other cellular compartments.
Overexpression artifacts: The overexpression of fusion proteins can lead to non-specific interactions or affect cellular physiology.
Technical complexity: The assay requires careful construction of fusion proteins and optimization of transfection conditions, which can be technically challenging and time-consuming.
Selection of Vectors: Choose appropriate vectors with the necessary domains for fusion to the proteins of interest—one vector should carry the DNA-binding domain and the other, the activation domain.
Cloning of Target Proteins: Clone the genes encoding the proteins of interest into these vectors, ensuring that they are correctly fused to either the DNA-binding or activation domain.
Selection of Reporter Gene: Select a suitable reporter gene that will indicate protein-protein interaction. Common choices include luciferase or GFP, which are easy to quantify.
Cell Line Selection: Choose a mammalian cell line that is suitable for transfection and in which the native context of the protein interactions can be maintained.
Transfection and Optimization: Transfect the cells with the constructs and optimize the conditions to maximize expression while minimizing toxicity or other negative effects on the cells.
Protein Interaction Mapping: Identifying interaction partners for a protein of interest can help in mapping interaction networks within cells.
Mutation Analysis: Assessing how mutations affect protein-protein interactions crucial for understanding the molecular basis of diseases.
Drug Discovery: Screening for molecules that disrupt or enhance specific protein-protein interactions, which can lead to the development of new therapeutics.
Functional Annotation: Helping to determine the function of uncharacterized proteins by identifying their interaction partners.
The two assays are similar in their basic principle of detecting protein-protein interactions through reporter gene activation. However, there are significant differences:
Cellular Environment: The M2H assay is conducted in mammalian cells, while the Y2H assay uses yeast cells. This difference is crucial as it allows the mammalian two-hybrid assay to mimic more accurately the natural environment and post-translational modifications of mammalian proteins.
Complexity of the System: Mammalian cells are generally more complex than yeast cells, with more extensive systems for protein modification and regulation, which can affect protein interactions.
Technical Challenges: Mammalian systems are often more challenging and expensive to maintain, and transfection efficiencies can vary, which may impact the sensitivity and reliability of the assay.
Protein Expression Levels: Overexpression of proteins can lead to non-specific interactions or artifacts. It's crucial to control expression levels to approximate physiological conditions as closely as possible.
Protein Folding and Post-Translational Modifications: Proper folding and post-translational modifications are essential for the natural function and interaction of proteins. In some cases, the mammalian cell machinery may not correctly modify or fold a recombinant protein.
Cell Health and Viability: The health of the cell line used can impact the assay, as stressed or unhealthy cells may not accurately represent normal physiological processes.
Choice of Reporter Gene and Sensitivity: The sensitivity of the reporter gene and the detection method can also influence the results. Choosing a highly sensitive reporter system can help in detecting weaker interactions more reliably.
Optimization of Expression Vectors: Use vectors with strong, mammalian-specific promoters to enhance protein expression. Also, choosing the appropriate vector backbone can improve stability and integration into host DNA.
Codon Optimization: Modify the coding sequences of the genes to match the codon usage preferred by the host mammalian cells, which can significantly enhance the expression of the recombinant proteins.
Co-expression with Molecular Chaperones: Co-transfect cells with vectors expressing molecular chaperones that assist in protein folding and stability, which can be particularly helpful for proteins that do not fold well in mammalian cells.
Adjustment of Culture Conditions: Modify cell culture conditions such as temperature, serum concentration, and cell density to create an environment that promotes better expression and stability of the proteins.
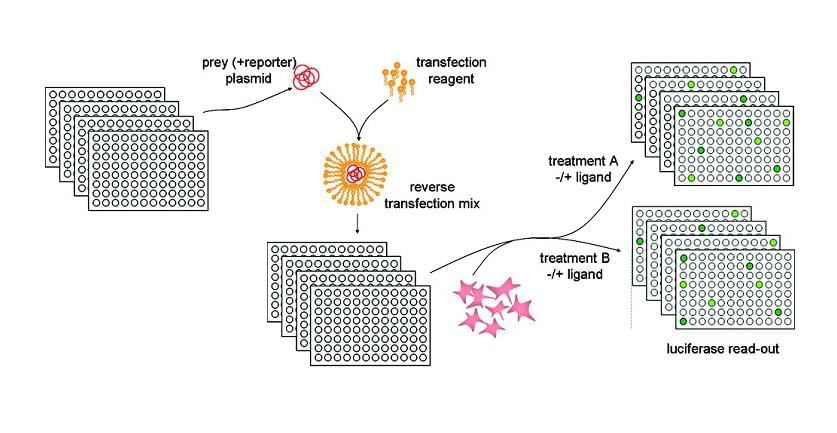
Mammalian two-hybrid assays can be adapted to study complex interactions involving more than two proteins. This is achieved by using additional fusion constructs and either multiple reporter genes or a single reporter gene with multiple response elements. For example, a third protein can be fused to another domain that influences transcriptional activation or repression in response to the interaction of the first two proteins. This adaptation allows researchers to explore dynamic protein networks and the effects of co-regulatory proteins in a mammalian cell context, providing a more comprehensive view of cellular function and signaling pathways.
Automation and Miniaturization: The assay must be adapted to a format suitable for automated handling and processing, typically in a microplate format. This requires miniaturization of the assay components and optimization of transfection protocols to work reliably in small volumes.
Consistency and Robustness: Ensuring that the assay is robust and consistent across many wells and plates is crucial. Variability in cell culture conditions, transfection efficiency, and reporter gene expression can affect the reliability of the assay.
Data Handling and Analysis: High-throughput screening generates large volumes of data. Developing efficient data processing and analysis tools to handle and interpret this data accurately is essential, especially for distinguishing true positives from false positives.
Cost Considerations: The use of mammalian cells and the necessity for specific reagents and equipment can make HTS using the M2H assay more expensive compared to other methods like the yeast two-hybrid system.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
